Join ADC San Diego with DetaiBio!
Over 800 world-leading ADC experts are invited to join the 14th World ADC San Diego. This meeting has been designed to optimize the therapeutic window of ADCs and accelerate their transformation into life-altering frontline treatments for oncology patients. Whether individuals are new to the ADC field or seasoned experts, this event represents the largest gathering of the ADC community and is an essential opportunity not to be missed.
The 14th World ADC San Diego covers all stages of ADC development, from early discovery to late-stage manufacturing and regulatory hurdles. Attendees can expect comprehensive coverage and support throughout their ADC journey.

Conference Informantion
Conference Name: ADC San Diego 2023
Conference Time: October 16-19, 2023
Conference Venue: Sheraton San Diego Hotel & Marina, San Diego, CA
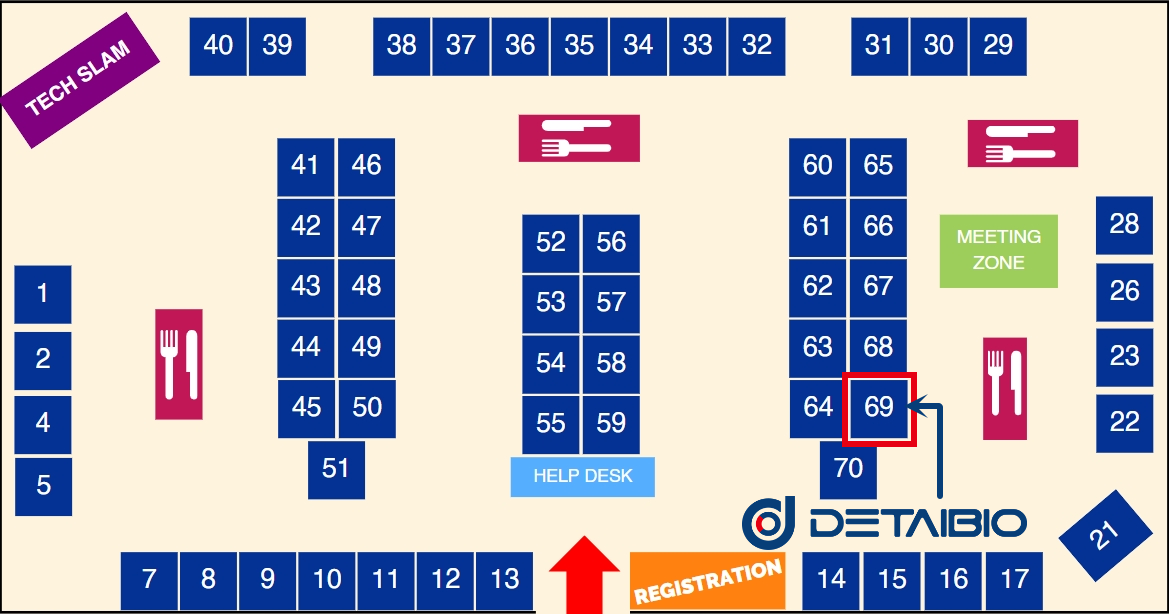
DetaiBio Booth: #69
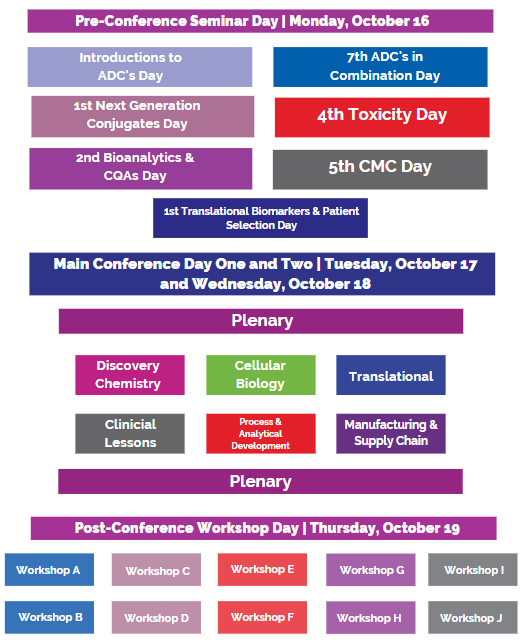
Conference Agenda (partial listed)
Whole List: https://worldadc-usa.com/program/conferences-days/conference-day-one/
About Us
Detai Bioscience, Inc. (“DetaiBio”), is a CRO vendor focusing on antibody discovery and functional protein research field. DetaiBio is aiming to provide high quality and economic service to speed up life science for our clients in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us

Applications of Antibodies from Different Animal Sources
Antibodies, also known as immunoglobulins, are essential tools in biomedical research, diagnostics, and therapeutics. While humans produce antibodies, various animal species also generate unique antibodies with distinct properties. These animal-derived antibodies have found diverse applications across different fields.
Mouse Antibodies:

Mouse monoclonal antibodies have been extensively employed in research and diagnostics for several decades. They are highly specific and have a wide range of applications, including Western blotting, ELISA, immunohistochemistry, and flow cytometry. Mouse antibodies are valuable tools for studying various diseases, including cancer, infectious diseases, and autoimmune disorders.
Rabbit Antibodies:

Rabbits produce high-affinity antibodies due to their unique immune system. Their antibodies are commonly used in research and diagnostics, especially in immunohistochemistry and immunofluorescence assays. Additionally, rabbit monoclonal antibodies are valuable tools for detecting and quantifying specific proteins in Western blotting and ELISA.
Goat and Sheep Antibodies:

Polyclonal antibodies from goats and sheep are widely used in different research applications. They are often employed in protein purification, immunoprecipitation, and flow cytometry. Due to their ability to recognize multiple epitopes, goat and sheep antibodies are also used in sandwich ELISA assays for detecting and quantifying analytes.
Camel Antibodies (Nanobodies):

Camels produce unique antibodies known as nanobodies. These single-domain antibodies have a smaller size and unique structure, allowing them to access hidden epitopes on antigens. Nanobodies are valuable tools in research, diagnostics, and therapeutics. They can be easily produced in bacteria and display excellent stability, making them suitable for applications in harsh conditions.
Shark Antibodies:

Sharks produce antibodies called IgNAR (Immunoglobulin New Antigen Receptor), which have a unique structure and exceptional stability. These antibodies have shown promise in therapeutic applications, including cancer treatment and targeted drug delivery. Their small size and robust nature make them valuable tools in biotechnology and diagnostics.
Chicken Antibodies:

Chicken antibodies, known as IgY, have unique properties that make them suitable for certain applications. They have a long half-life, high specificity, and low cross-reactivity with mammalian antigens. Chicken antibodies are used in research, particularly in immunodetection assays, such as ELISA and Western blotting. They are also emerging as promising tools in therapeutic applications, including cancer treatment and targeted drug delivery.
Conclusion:
Antibodies derived from different animal sources offer a broad range of applications in biomedical research, diagnostics, and therapeutics. Each animal species produces antibodies with specific characteristics that make them valuable tools for various assays and studies. Whether it's the high-affinity antibodies from rabbits, the multipurpose polyclonal antibodies from goats and sheep, the versatile mouse monoclonal antibodies, the unique nanobodies from camels, the robust IgNAR antibodies from sharks, or the specific IgY antibodies from chickens, these animal-derived antibodies continue to drive advancements in scientific research and medical applications.
CAR-T Therapy: Revolutionizing Cancer Treatment
Background:
Cancer is a devastating disease that affects millions of people worldwide. Despite advancements in medical science, the cure for cancer has remained elusive. However, recent breakthroughs in immunotherapy have given hope to patients and researchers alike. One such breakthrough is CAR-T therapy, which stands for chimeric antigen receptor T-cell therapy. This innovative treatment has shown remarkable success in treating various types of cancer and has the potential to revolutionize cancer treatment as we know it.
Understanding CAR-T Therapy:
CAR-T therapy is a type of immunotherapy that harnesses the power of a patient's own immune system to fight cancer. It involves the extraction of T-cells, which are a type of white blood cell responsible for recognizing and destroying abnormal cells in the body. These T-cells are then modified in a laboratory to express a chimeric antigen receptor (CAR) on their surface.
CAR-T therapy is a type of immunotherapy that harnesses the power of a patient's own immune system to fight cancer. It involves the extraction of T-cells, which are a type of white blood cell responsible for recognizing and destroying abnormal cells in the body. These T-cells are then modified in a laboratory to express a chimeric antigen receptor (CAR) on their surface.
Success Stories:
CAR-T therapy has shown remarkable success in the treatment of certain types of cancer, particularly hematological malignancies such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). In fact, CAR-T therapy has achieved complete remission rates of up to 90% in patients with relapsed or refractory ALL.
One of the most well-known success stories of CAR-T therapy is that of Emily Whitehead, who was diagnosed with ALL at the age of six. Conventional treatments failed to cure her, and her prognosis was grim. However, she became the first pediatric patient to receive CAR-T therapy in 2012. Today, Emily is cancer-free and living a normal life, thanks to the groundbreaking treatment.
Challenges and Limitations:
While CAR-T therapy holds immense promise, it also faces several challenges and limitations. One major challenge is the high cost of treatment. Currently, CAR-T therapy is expensive, making it inaccessible to many patients. However, with ongoing research and development, it is hoped that the cost will decrease in the future, making it more widely available.
Another limitation is the potential for severe side effects. CAR-T therapy can lead to a condition called cytokine release syndrome (CRS), where the immune system goes into overdrive, causing flu-like symptoms, fever, and in severe cases, organ damage. However, medical professionals are continuously working on improving the safety profile of CAR-T therapy to minimize these side effects.
Future Directions:
The success of CAR-T therapy has paved the way for further advancements in the field of immunotherapy. Researchers are exploring the use of CAR-T therapy in solid tumors, such as breast, lung, and pancreatic cancer, which have traditionally been more challenging to treat. Additionally, efforts are being made to develop off-the-shelf CAR-T cells, which can be readily available for patients without the need for individualized manufacturing.
Conclusion:
CAR-T therapy has emerged as a game-changer in the field of cancer treatment. Its ability to harness the power of the immune system to specifically target and eliminate cancer cells has shown remarkable success in clinical trials. While challenges exist, ongoing research and development are expected to overcome these limitations and make CAR-T therapy more accessible and safer for patients. With its potential to revolutionize cancer treatment, CAR-T therapy offers hope to countless individuals and their families who are battling this devastating disease.
What are the main techniques currently used to obtain monoclonal antibodies?
Monoclonal antibodies (mAb) are highly specific and functionally far superior to polyclonal antibodies (pAb), and are one of the most important types of biologics in the pharmaceutical market, widely used as highly specific diagnostic tools, and therapeutic drug development.
1) Hybridoma technology
Hybridoma technology was the first technology developed for the isolation of mAb. Since its development has revolutionized the discovery of mAb. The principle of the technique is to immortalize short-lived B cells by fusion with myeloma cells to produce a monoclonal immortalized hybridoma cell line, which is then screened for antigen-specific clones in its supernatant and further subcloned for cycling to produce strict monoclonality, usually subcloned for 2 to 3 cycles.
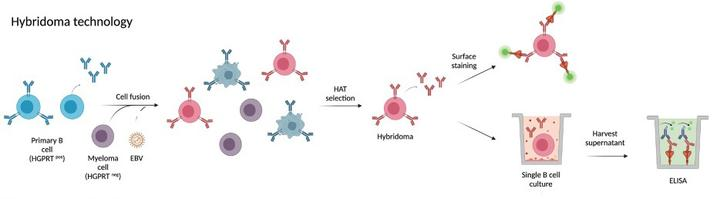
Fig.1 Process of hybridoma technology
Disadvantages:Hybridoma antibody development has a long cycle, excluding immunization, which takes approximately 4-6 months. Limited by the hybridoma fusion rate, only a small portion of B cells in the entire B cell population complete the fusion and the vast majority are lost, making it unsuitable for comprehensive screening of large antibody libraries. In addition, there is competition between different hybridomas in the same culture, and some non-secreting or low-secreting clones may exhibit faster growth rates than high-secreting clones.
2) Display technology
Methods of discovering and selecting monoclonal antibodies also include display techniques such as phage and yeast display. Currently phage display has been successfully used to screen a large number of antibody libraries. The principle of phage display technology is to insert a segment of exogenous gene into the appropriate position of the structural gene of phage shell protein. Under the condition that the reading frame is normal and does not affect the normal function of the shell protein, the exogenous gene will be expressed along with the expression of the shell protein, which results in the presentation of the polypeptide or protein on the surface of phage in the form of fusion protein
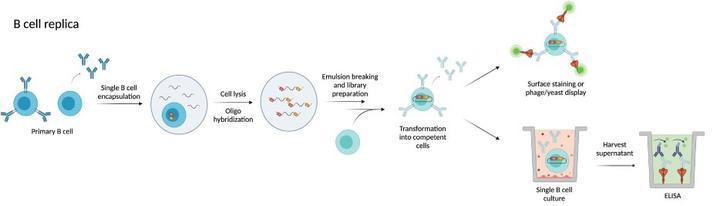
Fig.2 Process of display technology
DISADVANTAGES:It is critical to obtain natural VH-VL pairs in antibody development, however, demonstration techniques often rely on random combinations to form unnatural VH-VL antibody pairs. It has been suggested that the theoretical human B-cell library is 1012-1018, but the actual number of different B-cell clones in vivo is about 1 × 107 -2 × 107 due to sample size, out-of-frame mutations inserted during V(D)J recombination, and self-reactive BCR deletion, whereas in display technologies such as phage, the size of initial B-cell libraries is usually larger than 109, the number far exceeds that of natural B cell libraries, and the proportion of VH-VL unnatural matches is large.
3) Single B-cell Technology
Primary antigen-specific B cells are the main source of antigen-specific mAb, and single B-cell antibody technology allows for the direct isolation of single B cells from peripheral blood or lymphoid tissues in either a random or antigen-selective manner. Random B-cell isolation allows cell selection by micromanipulation, laser capture microdissection, and fluorescence-activated cell sorting (FACS). Antigen selection can be performed by screening antigen-specific B cells using antigen-coated magnetic beads, fluorescent dye-labeled antigens for multiparametric FACS, hemolytic plaque assays, and fluorescent focusing.
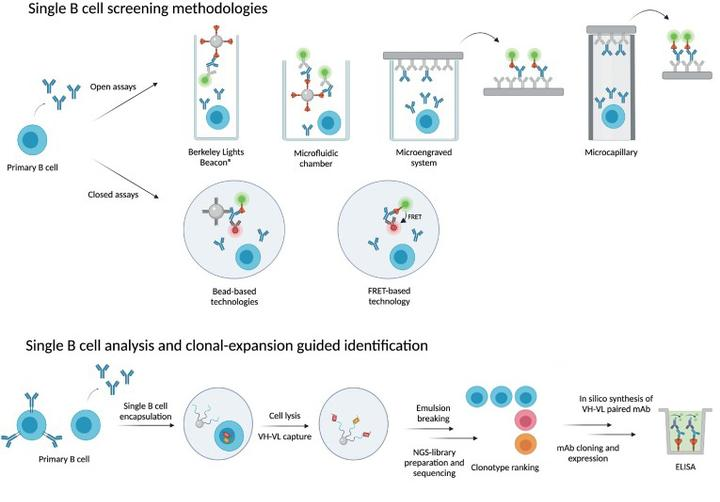
Fig3. Process of single B-cell antibody technology
Advantages of Single B-Cell Technology
The Single B-Cell Antibody Technology uses a relatively small number of cells to obtain a specific monoclonal antibody and is extremely efficient in mAb development. Single B-cell antibody technology can isolate functional mAb with conformational determinants that are difficult to mimic in vitro.FACS technology can also clearly distinguish the developmental and differentiation stages of B cells to be sorted based on the expression pattern of specific cell surface markers, and B cells at almost any stage of development can be sorted. The mAb obtained through single B cells, which are naturally matured in vivo, have a fairly high specificity for the target. In addition, the single B-cell antibody technology allows human antibodies to be obtained directly from human specimens or fully human transgenic mouse PBMCs, which is convenient and far easier and more effective than humanization via mAb from other species (e.g., mice, rats, and rabbits).
DetaiBio provides the one-stop antibody discovery service with an innovative self-developed Single B cell platform. DetaiBio SingleB® can get hits from both memory and plasma B cells of multiple species. Our in-house developed single B cell PCR kit that retrieves antibody V regions of the maximal diversity, within very short timeline-from immunization to hits: as fast as 29 days (mouse) and 49 days (rabbit). These advantages and other key features make desired antibody generation in DetaiBio happen in a fast-and-easy way. The excellent CDR diversity of obtained antibodies maximizes the possibility of getting candidate(s) robust enough to survive antibody drug development procedures that are full of risks of many kinds, or smart enough to well meet other unique or unusual application needs.
SingleB® Fast Antibody Discovery Technology
High efficiency: as fast as 29 days, saving 120 days compared to conventional hybridomas.
"1+1" Dual Screening: Memory B-cell + Plasma Cell Dual Screening with Rich Antibody Diversity.
Pre-screening:DeepLight® On-chip Cell Screening technology enables pre-screening of antibody function.
Natural maturation:the antibody is naturally matured in vivo with natural VH-LH pairing.
Comprehensive testing:Antibodies are subjected to Affinity Ranking, FACSBinding/Blocking and other multiple tests.
More choices: multiple species are available such as normal mice, rabbits, alpacas, and fully-humanized transgenic mice.
Reference
[1] Tiller T. Single B cell antibody technologies. Nat. Biotechnol. 2011, 28(5): 453-7.
[2] Marasco W.A., Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25: 1421–1434.
[3] Alessandro Pedrioli, Annette Oxenius. Single B cell technologies for monoclonal antibody discovery. Trends in Immunology. 2021, 42(12): 1143-1158.
[4] Kuppers R. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993, 12: 4955–4967.
[5] Obiakor H. A comparison of hydraulic and laser capture microdissection methods for collection of single B cells, PCR, and sequencing of antibody VDJ. Anal. Biochem. 2002, 306: 55–62.
[6] Wardemann H. Predominant autoantibody production by early human B cell precursors. Science. 2003, 301: 1374–1377.
DetaiBio invites you to the Immuno-Oncology Summit!
11th Annual Immuno-Oncology Summit will be held in Seaport Hotel,Boston, MA. The CHI Immuno-Oncology Summit has emerged as the premier gathering focused on the newest biotherapeutics, technological advancements, and the beneficial sharing of high-caliber research from all areas of immuno-oncology. Participants in CHI's IO Summit have access to a thorough three-day program that allows them to assess bi- or multi-specific biotherapeutics, investigate the most recent advances in established and emerging cellular therapies, develop predictive preclinical models for translational strategies, and evaluate AI and computational tools for the identification of new targets, target classes, and combinations to overcome resistance.

Conference Informantion
Conference Name: Immuno-Oncology Summit
Conference Time: August 7-9, 2023
Conference Venue: Seaport Hotel,Boston, MA
DetaiBio Booth: #17
Conference Agenda (partial listed)
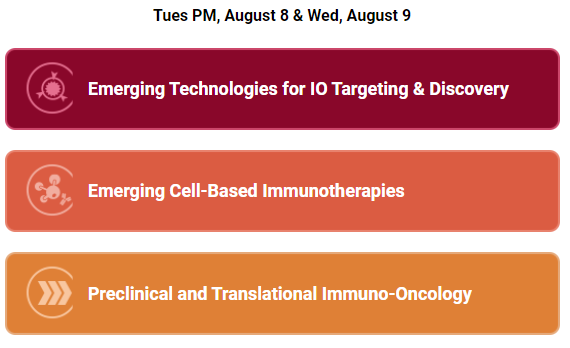
Whole List: https://www.immuno-oncologysummit.com/programs
About Us
Detai Bioscience, Inc. (“DetaiBio”), is a CRO vendor focusing on antibody discovery and functional protein research field. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
Take part in the PEGS Boston Conference & Expo with DetaiBio!
The PEGS Boston Summit will be held May 15-18 at the Hynes Convention Center in Boston. PEGS Boston Summit is the world's leading biologics event, which comprehensively covers the related fields of protein and antibody engineering, immunotherapy, expression and analysis of proteins and antibodies in oncology, and evaluation of the immunogenicity of biological macro-molecular drugs. It is recognized as one of the most important and leading international conferences in the bio-pharmaceutical industry.

Conference Informantion
Conference Name: PEGS Boston Conference & Expo
Conference May 15-18, 2023
Conference Venue: Hynes Convention Center, 900 Boylston St, Boston, MA
DetaiBio Booth: #113
Conference Agenda (partial listed)
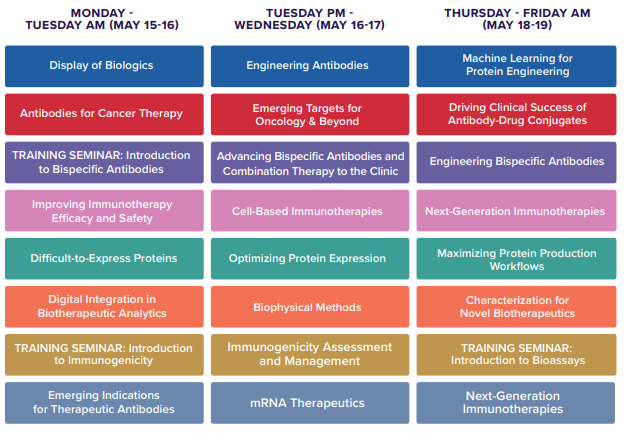
Whole List: https://www.pegsummit.com/conference-streams
About Us
Detai Bioscience, Inc. (“DetaiBio”), is a CRO vendor focusing on antibody discovery and functional protein research field. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
Attend 2023 Chinese Antibody Annual Conference with DetaiBio!
The 2023 Chinese Antibody Annual Conference will be held on Sunday, May 14, 2023, in Cambridge, Massachusetts, USA. The theme of this year's annual meeting is "PostCOVID-19 Pandemic: New Frontiers and Opportunities of Antibody-based Biologics". This conference will focus on the frontiers of therapeutic antibodies and innovative technologies for antibody discovery, development and manufacturing. The CAS 2023 Annual Conference will bring together experts and scholars to discuss the difficulties in antibody drug development and share the latest technology and research results.

Conference Informantion
Conference Name: 2023 Chinese Antibody Annual Conference
Conference Date: May 14, 2023
Conference Venue: Boston Cambridge Marriott Hotel, 50 Broadway, Cambridge, MA 02142, USA
DetaiBio Booth: #25
Conference Agenda (partial listed)

About Us
Detai Bioscience, Inc. (“DetaiBio”), is a CRO vendor focusing on antibody discovery and functional protein research field. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
Fully Humanized Monoclonal Antibody Discovery Technology (Transgenic Mice)
The experimental principle for the discovery of fully humanized antibodies in transgenic mice is that antibodies are not generated with wild type mice, but with mice in which murine antibody-coding genes have been replaced by human ones. Successful production of fully humanized antibodies requires that human antibody gene fragments must be precisely integrated, efficiently rearranged and expressed in host mice and that these fragments interact with murine immune system signaling mechanisms, therefore allowing human antibody gene fragments to be selected, expressed and secretedby B cells upon antigen stimulation in mice. The basic approach is to use homologous recombination in murine embryonic stem (ES) cells to make the original murine gene deletion, and then transfer the reconstructed human antibody germline gene microsites into mice by microinjection and other techniques, and finally secrete the whole human sequence of antibody from the hybridoma of mAb.
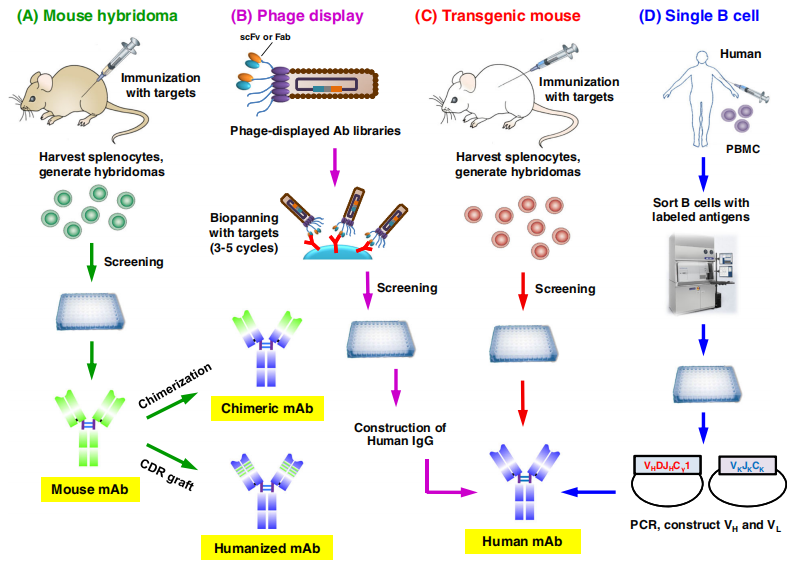
Figure 1. Approaches for the development of therapeutic antibodies
Advantages and Disadvantages of xxx
The main advantage of getting fully human antibodies from transgenic mice is efficacy that is superior to other techniques of producing anti-normal human protein mAb, since transgenic mouse's system of recognizing antigen and producing antibodies remains intact and readily recognizes the human protein as a foreign body. Moreover, since the antibodies are produced in vivo, they undergo a normal assembly and maturation process, thus ensuring that the resulting product has high target binding affinity.
However, there are several disadvantages with transgenic mouse technology at present. First, immune tolerance remains a problem. Although immune response can be enhanced by adjuvants and tailored immunization methods, it is still difficult to obtain high-affinity antibodies to some human antigens. Second is the interference of murine antibodies. Thirdly, it is difficult to immunize against toxic antigens.
The Major Platforms of Fully Human Antibody Mice
| Company | Product | hVHa | hVKb | Constant | Country |
|---|
| Medarex | HuMabMouse | 4 | 4 | Human(Cμ) | US |
| Abgenix | XenoMouse | 17 | 17 | Human(Cμ-Cδ-Cγ2) | US |
| Ligand | OmmiRat | 22 | 12 | Rat | US |
| Kymab | KyMouse | 43 | 37 | Mouse | US |
| Regeneron | VelocImmune | 47 | 23 | Mouse | US |
| Harbour Antibodies BV | H2L2Mouse | 18 | 11 | Mouse | US |
| Trianni | Trianni Mouse | 44 | 39 | Mouse | US |
| Immunocan | Immuno Mouse | 50 | 40 | Mouse | US |
a The number of human heavy chain variable region
b The number of human kappa chain variable region
FDA-Approved Human mAbs (Partial Listed)
With continuous optimization and upgrading in development and application, the global transgenic mouse platforms have generated dozens of antibody drugs approved by FDA so far.
| No. | Antibody | Brandname | Company | Approval* | Target | Technology |
|---|
| 1 | Panitumumab | Vectibix | Amgen | 2006 | EGFR | XenoMouse |
| 2 | Ustekinumab | Stelara | Johnson & Johnson | 2009 | IL-12 | HuMabMouse |
| 3 | Canakinumab | Ilaris | Novartis | 2009 | IL-1β | HuMabMouse |
| 4 | Golimumab | Simponi | Johnson & Johnson/Merck | 2009 | TNFα | HuMabMouse |
| 5 | Denosumab | Prolia, Xgeva | Amgen | 2010 | RANKL | XenoMouse |
| 6 | Ipilimumab | Yervoy | Bristol-Myers Squibb | 2011 | CTLA-4 | HuMabMouse |
| 7 | Nivolumab | Opdivo | Bristol-Myers Squibb | 2014 | PD-1 | HuMabMouse |
| 8 | Alirocumab | Praluent | Sanofi and Regeneron | 2015 | PCSK9 | VelocImmune Mouse |
| 9 | Daratumumab | Darzalex | Johnson & Johnson (Genmab) | 2015 | CD38 | HuMabMouse |
| 10 | Evolocumab | Repatha | Amgen | 2015 | PCSK9 | XenoMouse |
| 11 | Secukinumab | Cosentyx | Novartis | 2015 | IL-17α | XenoMouse |
| 12 | Olaratumab | Lartruvo | Eli Lilly | 2016 | PDGFRα | HuMabMouse |
| 13 | Dupilumab | Dupixent | Sanofi and Regeneron | 2017 | IL-4R | VelocImmune Mouse |
| 14 | Durvalumab | Imfinzi | Medimmune/ AstraZeneca | 2017 | PD-L1 | XenoMouse |
| 15 | Sarilumab | Kevzara | Sanofi and Regeneron | 2017 | IL-6R | VelocImmune Mouse |
| 16 | Erenumab | Aimovig | Novartis and Amgen | 2018 | CGRPR | XenoMouse |
| 17 | Cemiplimab | Libtayo | Regeneron | 2018 | PD-L1 | VelocImmune Mouse |
*Year of the first US FDA approval
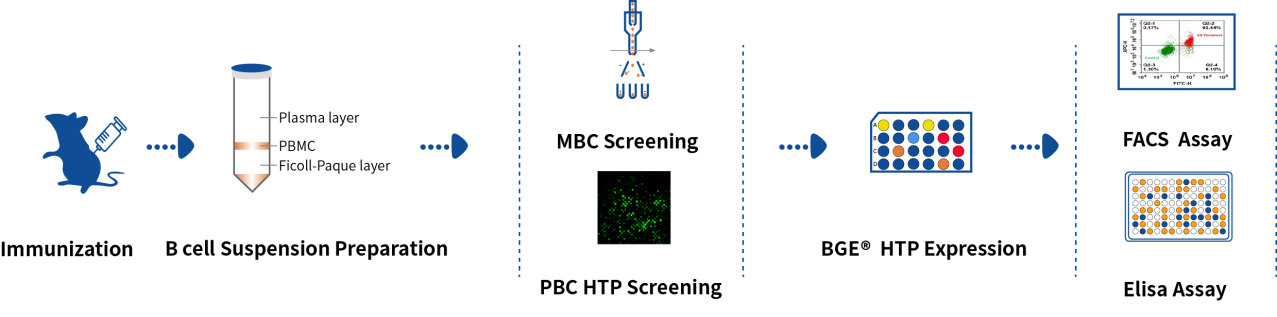
Hu-Mouse SingleB mAb Discovery Service
DetaiBio has partnered with Immunocan use ImmuMab Mouse. ImmuMab Mouse is the first human antibody animal platform created by MASIRT technology, achieving Mb-scale large fragment gene replacements. MASIRT allows intact human immunoglobulin variable region genes to be encompassed, with the largest genetic humanization ever achieved in mouse immunoglobulin variable loci (in situ).
With ImmuMab Mouse and SingleB Antibody Discovery Platform combined, DetaiBio is able to provide Hu-Mouse SingleB mAb Discovery service by directly screening antigen-specific memory B cells and plasma cells hu-mice. From immunization to obtaining monoclonal antibodies, the whole process can be completed in as short as 49 days, with high affinity and fully humanized antibodies delivered.
- Time-saving: from shots to hits, 49 days.
- Highly Diverse: hits from both PBC & MBC, retrieve maximal diversity.
- Fully humanized: Intact target affinity.
- Cost-effective: friendly sub-licensing agreements.
Reference
1.Fujiwara, S. (2018). Humanized mice: a brief overview on their diverse applications in biomedical research. Journal of cellular physiology, 233(4), 2889-2901.
2.Lu, R. M., Hwang, Y. C., Liu, I. J., Lee, C. C., Tsai, H. Z., Li, H. J., & Wu, H. C. (2020). Development of therapeutic antibodies for the treatment of diseases. Journal of biomedical science, 27(1), 1-30.
3.Yin, L., Wang, X. J., Chen, D. X., Liu, X. N., & Wang, X. J. (2020). Humanized mouse model: a review on preclinical applications for cancer immunotherapy. American Journal of Cancer Research, 10(12), 4568.
Attending AACR Annual Meeting 2023 with DetaiBio!
The AACR Annual Meeting is the focal point of the cancer research community, where scientists, clinicians, other health care professionals, survivors, patients, and advocates gather to share the latest advances in cancer science and medicine. From population science and prevention; to cancer biology, translational, and clinical studies; to survivorship and advocacy; the AACR Annual Meeting highlights the work of the best minds in cancer research from institutions all over the world.

Conference Informantion
Conference Name: AACR Annual Meeting 2023
Conference Date: April 14-19, 2023
Conference Venue: Orange County Convention Center, Orlando, Florida
DetaiBio Booth: #671
Conference Agenda (partial listed)

Whole List: https://www.aacr.org/meeting/aacr-annual-meeting-2023/schedule-at-a-glance/
About Us
Detai Bioscience, Inc. (“DetaiBio”), is a CRO vendor focusing on antibody discovery and functional protein research field. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
Different Approaches to The Development of Nanobodies
Nanobodies and Their Unique Advantages
A new type of antibody naturally found in camelid, such as alpacas, differs in structure from traditional antibodies. This antibody consists of only two heavy chains, called heavy-chain antibody (HCAb).It lacks light chain and CH1 structures in heavy chain but retains complete antigen-binding activity. This heavy-chain variable region, which can specifically bind antigen, is called single-domain antibodies (sdAbs), or Nanobody, or VHH, which is the smallest unit of the antigen binding fragment.
Compared to conventional antibodies, nanobodies have a small molecular weight and simple structure. Based on the advantage of small molecular weight, nanobodies furthermore have several features that make them show great potential for new drug discovery. Nanobodies exhibit high affinity, high specificity, strong penetration and ease of modification and expression, and the complete antibody sequence was easy to obtain. Meanwhile, nanobodies can be produced in high quality and stable by in vitro recombinant expression, effectively avoiding the problem of batch-to-batch variation of traditional antibodies.
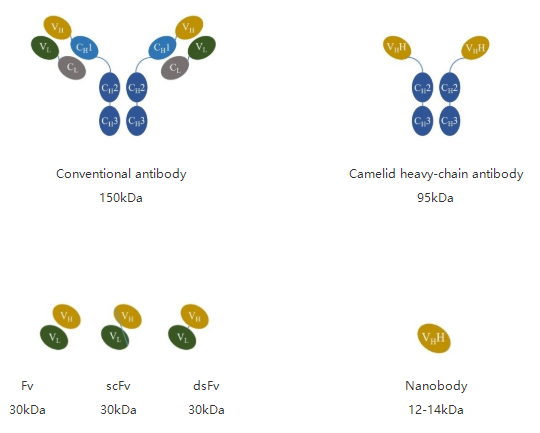
Figure 1. Different antibody types and molecular weights
Screening and Preparation of Nanobodies
Nanobodies are now commonly obtained through alpacas to obtain antibody genes, which are then screened by library display techniques to obtain the most suitable antibody sequences.
Based on phage display, the construction of a nanobody gene library is a technical pathway to obtain nanobody sequences. Phage libraries are mainly divided into natural library, immune library and synthesis library. Natural library constructs antibody genes from alpaca peripheral blood lymphocytes, bone marrow, spleen cells and tissues, and then clone these fragments directly into expression vectors for expression and display. Although natural library can avoid the time-consuming and laborious immunization process, they require larger library capacity and the affinity of the obtained nanobodies are most likely lower than that of immune libraries.
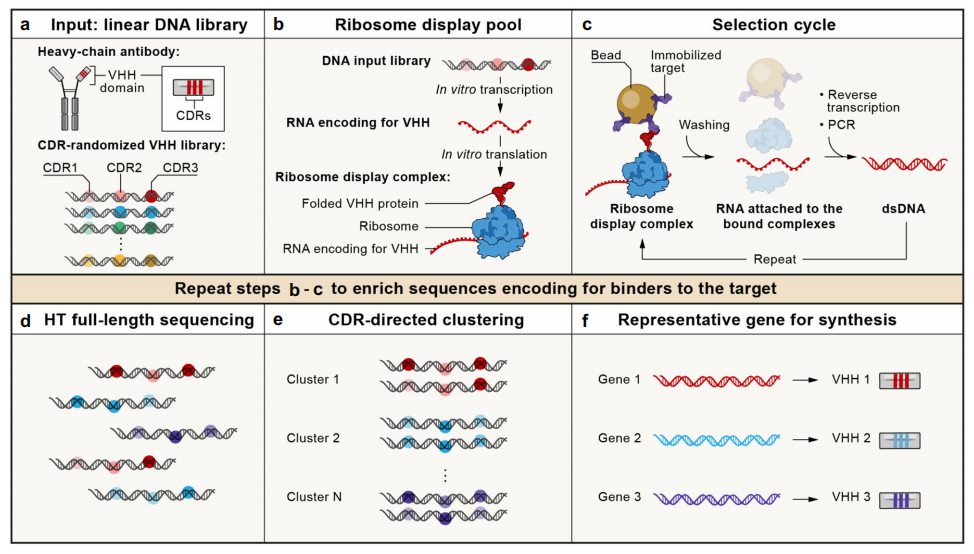
Figure 2. A cell-free nanobody engineering platform.
(Chen, Gentili, Hacohen & Regev, 2021)
To construct an immune library, albaca were immunized with antigens to extract lymphocytes containing mature B cells. The RNA of the B cells is extracted and inverted into a cDNA library, and the specific gene fragment is obtained by PCR amplification again in the cDNA library using specially designed primers. At last, subsequently, the nanobodies are displayed on the phage surface, and after the positive clones are sorted by liquid-phase or solid-phase, the diverse antibody sequences can be obtained by sequencing.
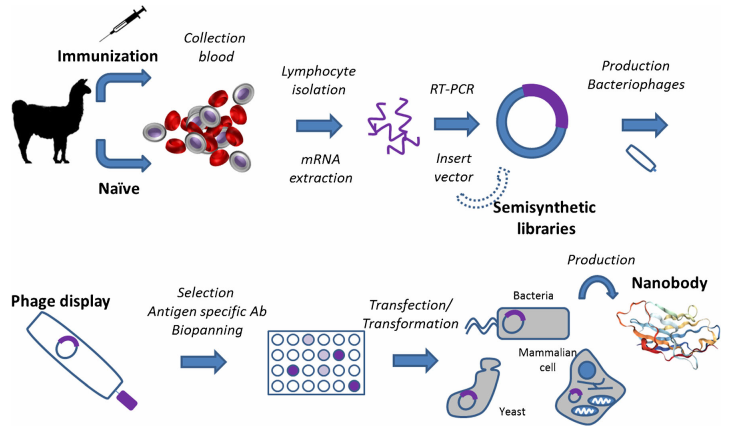
Figure 3. Nanobody production scheme using a phage display library.
(Salvador, Vilaplana & Marco, 2019)
The synthesis library is based on antibody sequences with good expression characters and strong stability as the framework to improve the expression level of antibody in the library and reduce the toxicity of the expressed products to the host cells. By increasing the proportion of functional antibody molecules in the antibody library, the real capacity of the antibody library can be increased. The construction of synthesis library does not require immunity, and can also be applied to non-immunogenic targets. However, the obtained nanobodies may require improved affinity and stability, and require very large library scale (109-1015).
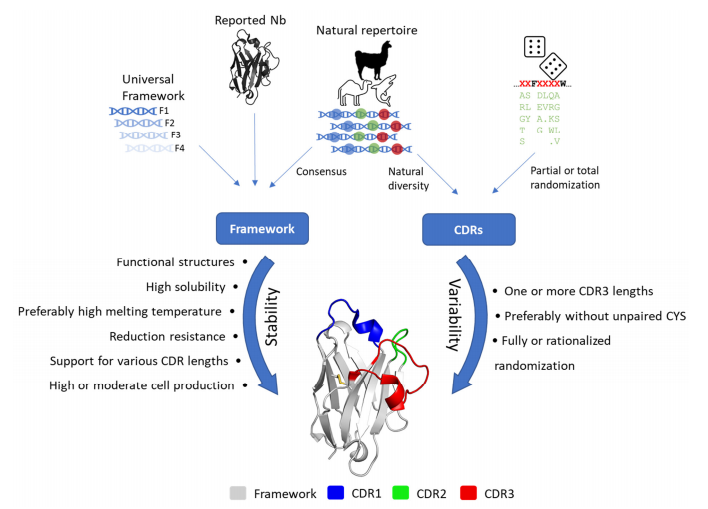
Figure 4. Strategy and desired characteristics for the generation of synthetic libraries.
(Ernest et al., 2022)
However, phage surface display technology is extremely dependent on antibody libraries with high library capacity and rich diversity, and phage manipulation is easy to contaminate, spread and difficult to control. Some laboratories also use other library display techniques, such as yeast display and ribosome display. The biggest drawback of these techniques is that there is not enough antibody diversity and a lot of antibody information is lost in the process of library preparation. Moreover, due to the steps of RNA extraction and reverse transcription, which are highly susceptible to contamination and degradation, it is difficult to ensure a HTP screening library capacity, so that the subsequent reverse transcription and PCR amplification are easily affected by the specificity and quantity of the template, resulting in screening failure or prolonged screening cycle.
Therefore, the technical path of obtaining nanobodies based on Single B cell cloning is a better choice. The Single B rapid screening platform is a short-term, simple, diverse, efficient and phage-free antibody screening method. It combines flow cell sorting and high-throughput on chip sequencing technologies to directly sort individual B cells and sequence their antibody-coding gene to obtain antibody sequence information. This method can cover all B cells targeting antigen specificity at one time, with good genetic diversity and high efficiency, and reduce the loss of comprehensive coverage of positive antibodies when constructing libraries. At the same time, it can avoid the problems of false positives and non-specific binding during the screening of monoclonal antibodies using phage libraries. Compared to the library building process, which requires a lot of time for panning, the Single B-cell screening process is often extremely fast, greatly reducing the project cycle.
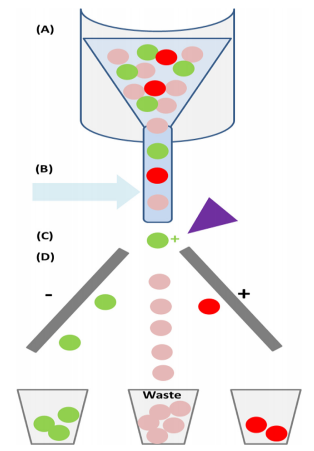
Figure 5. Fluorescence activated cell sorting (FACS).
(Fitzgerald & Leonard, 2017)
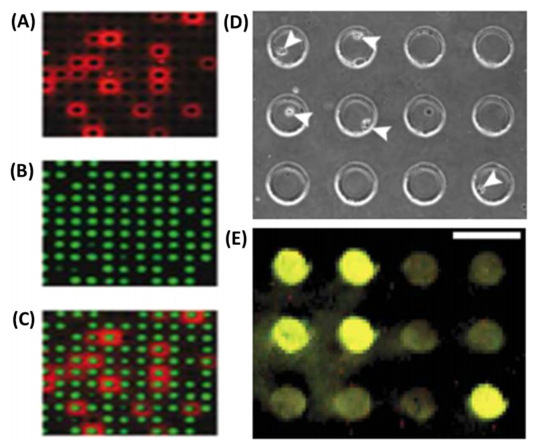
Figure 6. microengraved microwells.
(Fitzgerald & Leonard, 2017)
Comparison of Different Development Methods
| DetaiBio
SingleB® VHH | Phage Display Library |
|---|
| Synthesis Library | Natural Librar | Immune Library |
|---|
| Source of Antibody | Memory B cells and plasma cells | / | B cells, without immune animals | B cells, immune animals |
| Library Scale | - | 109-1015 | 109-1011 | 106-108 |
| Throughput | Very high | High | High | High |
| Screening Timeline | 1 day | 2 months | 2 months | 2 months |
| Antibody Diversity | High | Low | Low | Medium |
| Antibody Affinity | Very high | High | Low | High |
| Limitations | / | Depends on the bacteria in the correct transcription, translation, folding and assembly |
Alpaca SingleB® VHH Discovery Service
Based on the flow cytometry sorting platform and DeepLight® screening platform, DetaiBio provides alpaca VHH SingleB® rapid preparation service, from alpaca immunization, double screening of memory B cells and plasma cells, BGE® high-throughput expression, expression identification to purification, the whole process takes only 90 days, saving at least 15 days compared with immune display libraries.
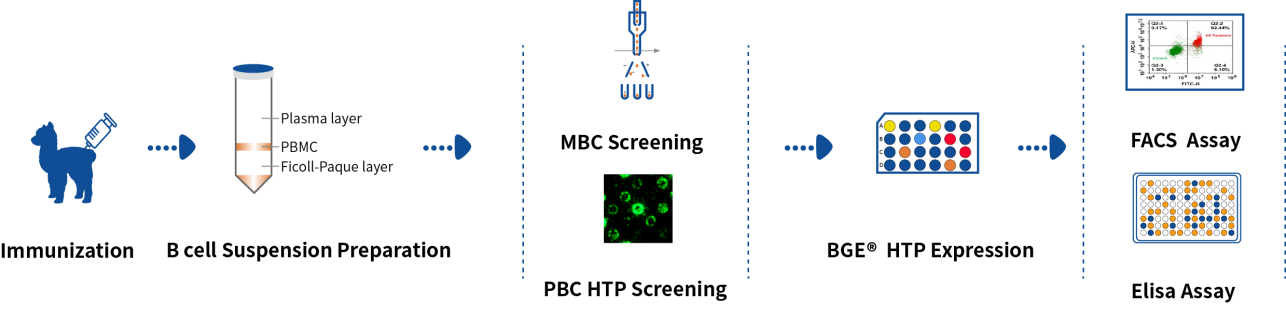
Conclusion
Overall, the unique nature of nanobodies greatly broadens their application scope, especially for some targets that are difficult to be addressed by traditional techniques, which creates a huge market opportunity for nanobodies. Compared with library display, SingleB® technology has many advantages, such as fast speed, large delivery, good diversity and high affinity, which can avoid the lack of diversity due to limited library capacity and greatly reduce the preparation time of nanobodies.
Reference
1.Chen, X., Gentili, M., Hacohen, N. and Regev, A., 2021. A cell-free nanobody engineering platform rapidly generates SARS-CoV-2 neutralizing Nature communications, 12(1), p.5506.
2.Fitzgerald, V. and Leonard, P., 2017. single cell screening approaches for antibody discovery. methods, 116, pp. 34-42.
3.Lin, J., Gu, Y., Xu, Y., Yu, J., Tang, J., Wu, L., Zhou, Z., Chen, C., Liu, M., Chun, X. and Liu, H., 2020. Characterization and applications of nanobodies against Pseudomonas aeruginosa Exotoxin A selected from single alpaca B cells. Biotechnology & Biotechnological Equipment, 34(1), pp.1028-1037 .
4.Lyu, M., Shi, X., Liu, X., Liu, Y., Zhu, X., Liao, L., Zhao, H., Sun, N., Wang, S., Chen, L. and Fan, L., 2022. Generation and Screening of Antigen-Specific Nanobodies from Mammalian Cells Expressing the BCR Repertoire Library Using Droplet-Based Microfluidics. Analytical Chemistry, 94(22), pp.7970- 7980.
5.Salvador, J.P., Vilaplana, L. and Marco, M.P., 2019. Nanobody: outstanding features for diagnostic and therapeutic applications. Analytical and bioanalytical chemistry, 411, pp. 1703-1713.
6.Valdés-Tresanco, M.S., Molina-Zapata, A., Pose, A.G. and Moreno, E., 2022. Structural insights into the design of synthetic nanobody libraries. Molecules, 27(7), p.2198.