Fine-tuned CD3 Affinity: Desired Balance between Antitumor Activity and Safety Drives the Design of Next-generation T Cell-engagers
Cytokine release-related syndrome (CRRS) is commonly observed in T cell engagers (TCEs) clinical trials. It is triggered often by excessive T cell activation, which in turn impairs desirable drug distribution and long-term efficacy.
Is cytokine production a hallmark of T cell activation? Or, in clinical setting, are high levels of cytokine production necessary for TCE efficacy?
The answer is NO, for both questions.
Studies unveiled that activation of biological functions in CTLs is determined by the molecular dynamics between CTLs and targets, and there exists an activation THRESHOLD for cytotoxicity advancing to full activation.
These seminal findings indicated that T cell killing does NOT require the formation of a stable mature immunological synapse, and have formed rationale underlying decoupling cytotoxicity from toxicity-triggering T cell stimulation in the development of next-generation TCEs, where CD3-binding affinity is finely tuned (generally, attenuated) to reach a "sweet spot" of signal that drives anti-tumor cytolytic activity without generating excessive cytokine release and/or T-cell exhaustion.
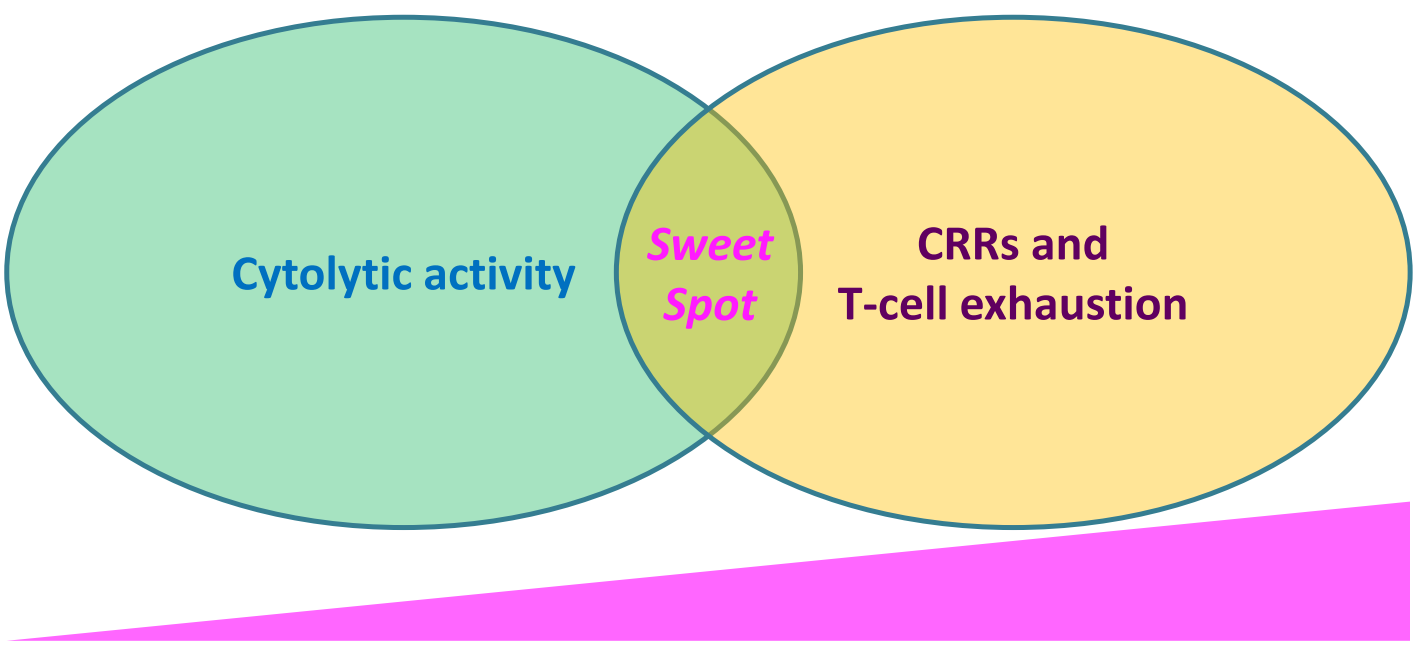
Figure 1.Fine-tuning CD3-binding affinity strikes a balance for optimal anti-tumor cytolytic activity, minimizing cytokine release and T-cell exhaustion
To date, quite a lot of novel TCEs have been built and investigated relying on the concept, and data produced so far support the "decoupling" theory, as exemplified by the developers and their candidate TCE drugs below.
- TeneoBio (TNB-486)
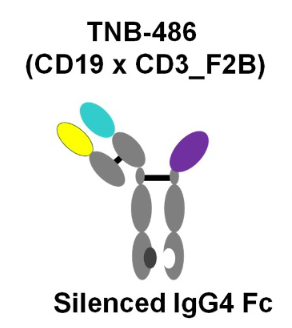
Figure 2. Structure of TNB-486
This CD19xCD3 bispecific antibody was designed for the treatment of B-NHL. Although its low affinity CD3-targeting arm lowered its in vitro killing potency, but it achieved the same maximum killing, and stimulated significantly less cytokine release. Amazingly, robust and long-term in vivo tumor cell killing occurred at low dose only. Favorable biodistribution or less propensity for inducing T-cell exhaustion or activation-induced cell death (AICD) resulting from low CD3 affinity may be responsible for its improved in vivo function.
This new TCE represented a novel therapeutic that induces potent T cell-mediated tumor-cell cytotoxicity uncoupled from high levels of cytokine release, making it an attractive candidate for B-NHL therapy.
- Genentech (HER2 TDB 1)
Figure 3.Structure of HER2 TDB 1
Bulit to lower systemic cytokine release and on-target/off-tumor toxicity to normal tissues of TCEs, this HER2/CD3 TCE of low binding affinity for CD3 proved that low affinity led to better overall tolerability in animal model, while T cell binding affinity had but very limited impact on in vitro and in vivo antitumor activity. The data indicated that fine-tuned affinity for CD3 as well as tumor target is a promising strategy for achieving maximal therapeutic index of CD3 TCEs.
- Amgen (AMG 424)
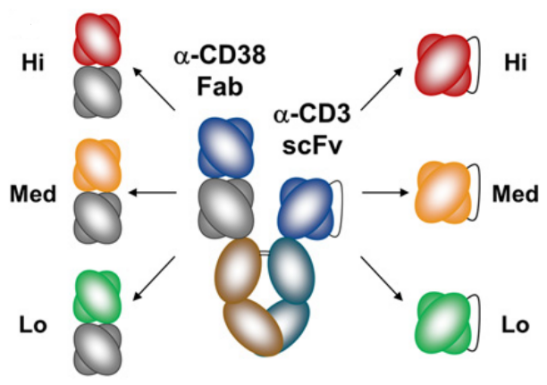
Figure 4. Structure of AMG 424
This CD3 affinity-optimized TCE showed ability to kill cancer cells and trigger T-cell proliferation, but with attenuated cytokine release, and its potential to elicit significant clinical activity in patients with multiple myeloma.
- Regeneron (REGN4018)
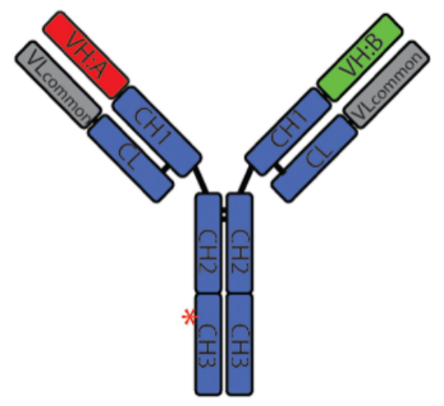
Figure 5. Structure of REGN4018
This CD3 affinity -customized MUC1xCD3 TCE for the treatment of ovarian cancer indicated attenuated profiles of cytokine release and favorable biodistribution, while its tumor killing potency remained, demonstrating potent antitumor activity and good tolerability of REGN4018, and is now under clinical evaluations.
- AbCellera
This biotech is Identifying TCEs with balanced anti-tumor potency and toxicities, mostly CRRS by fine-tuning CD3-binding affinity as well as disease target-binding moieties. Its proof-of-concept TCEs have showed high potency with low cytokine release.
Reference
1.Faroudi, Mustapha, et al. "Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold." Proceedings of the National Academy of Sciences 100.24 (2003): 14145-14150.
2.Riquelme, Erick, et al. "The duration of TCR/pMHC interactions regulates CTL effector function and tumor‐killing capacity." European journal of immunology 39.8 (2009): 2259-2269.
3.Malik-Chaudhry, Harbani K., et al. "TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL." MAbs. Vol. 13. No. 1. Taylor & Francis, 2021.
4.Staflin, Karin, et al. "Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody." JCI insight 5.7 (2020).
5.Zuch de Zafra, Christina L., et al. "Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell–recruiting antibody optimized for cytotoxicity and cytokine release." Clinical Cancer Research 25.13 (2019): 3921-3933.
6.Crawford, Alison, et al. "A Mucin 16 bispecific T cell–engaging antibody for the treatment of ovarian cancer." Science translational medicine 11.497 (2019): eaau7534.
With its cutting-edge single B antibody discovery platforms, DetaiBio is working on discovering high-quality, custom-made agonistic antibodies targeting stimulatory receptors of cytolytic effector cells for building next-generation multi-specifics, including T cell engagers and NK engagers. Visit us at www.detaibio.us to see how we can help upgrade your projects.








