TCR Targeted Tumor Antigens:
Prime Candidates for Cancer Immunotherapies
TCR-targeted cancer antigens, due to their high disease-specificity, have gained increased attention in recent years, especially in the treatment of solid tumors for which there has been a severe lack of “clean” targets for improved efficacy and safety profiles in clinical studies and practices.
A typical TCR structure of CD8+ T cells is illustrated as below.
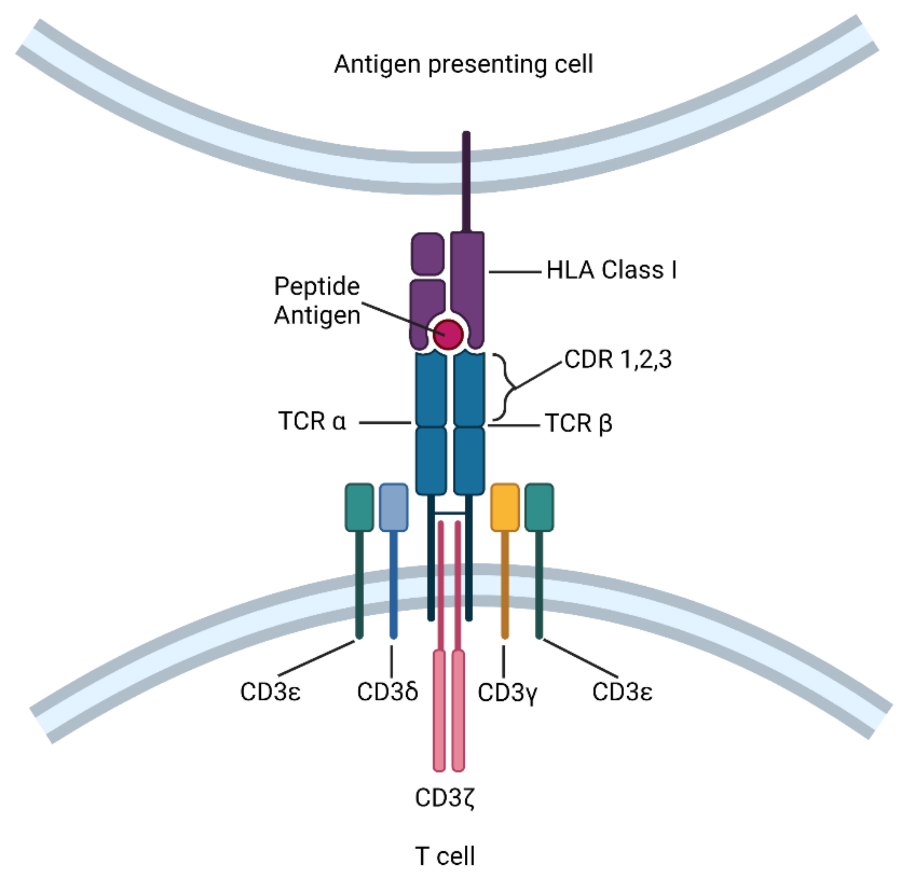
In recently years, significant progress has been achieved in identifying the precise and presentable cancer antigens, some of which, excitingly, have been clinically tested for mediating immune rejection of disease cells, as briefly summarized as follows.
1. Tumor-associated antigens (TAAs): they are, generally, expressed by tumor cells but also in some healthy tissues, potentially leading to on-target off-tumor toxicity. Among them are:
- 1) Differentiation antigens: expressed by cancer cells as well as normal cells of the same tissue origin, such as GP100; clinically appropriate to target when their expression is restricted to dispensable healthy tissues.
In early 2022, FDA approved KIMMTRAK by Immunocore, a TCR/ CD3 bispecific fusion protein for uveal melanoma treatment, targets presented GP100.2 Besides, KIMMTRAK is the first T cell engager drug ever approved for solid tumor treatment. This new class of T cell–redirecting bispecific fusion proteins, as illustrate below , use an engineered high-affinity TCR to target intracellular antigens presented as a peptide–HLA complex on target-cell surface. 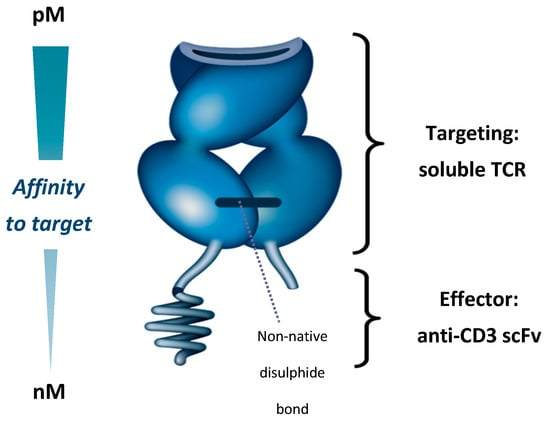
- 2) Overexpressed antigens: expressed at high levels in cancer cells but minimally expressed in healthy cells; the differential expression makes possible a therapeutic window.
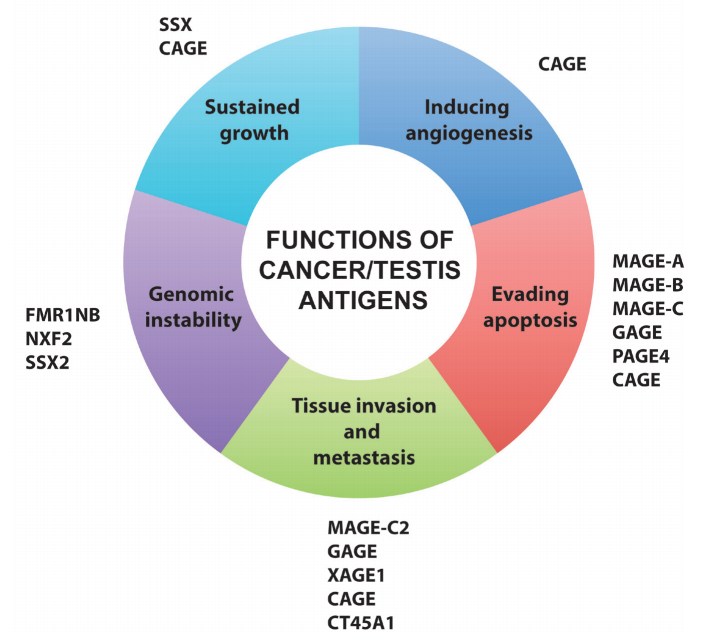
- 3) Cancer-germline antigens (CGAs): expressed in immune-privileged germline tissues (which lack antigen presentation machinery), while epigenetically silenced in somatic tissues; becoming aberrantly re-expressed in tumors to promote oncogenesis, thus greatly reducing the risk for on-target off-tumor toxicity. CGAs are being identified, as indicated below , and some of them have been investigated for cancer treatment studies.3
2. Tumor-specific antigens (TSAs): they are genetically encoded in cancer cells but not present in the genome of any normal cells, allowing for excellent therapeutic window.
1) Viral antigens: identified in viral oncogene driven cancers; but nearly absent in normal cells.
Ebvalla developed by Atara., a first ever allogeneic (off-the-shelf) T-cell therapy approved by EU indicated for a rare but deadly lymphoma, targets presented Epstein-Barr virus (EBV) viral oncoproteins
Although not a TCR therapy, its approval clinically validates the therapeutic values of viral antigens.
- 2) Neoantigens: expressed exclusively by cancer cells due to their genomic instability; attractive targets that would pose essentially no risk for on-target off-tumor toxicity.
While most neoantigens are ineffective, public neoantigens from driver mutations expressed homogenously and shared among patients are immunogenic and restricted to a common HLA.
KRAS G12V is an optimal example. AFNT-211, a TCR-T cell therapy targeting KRAS G12V-expressing solid tumors, is to enter clinical trial.
More breakthroughs are expected from cancer antigen-targeted TCR therapies.
Precision makes efficacy and safety.
DetaiBio is leveraging its single T cell sorting platform and single cell RT-PCR technology to unlock its TCR discovery-based therapy research. Please contact us for potential collaboration.
Reference
1. Sun, Y., Li, F., Sonnemann, H., Jackson, K. R., Talukder, A. H., Katailiha, A. S., & Lizee, G. (2021). Evolution of CD8+ T cell receptor (TCR) engineered therapies for the treatment of cancer. Cells, 10(9), 2379.
2. Gjerstorff, M. F., Andersen, M. H., & Ditzel, H. J. (2015). Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget, 6(18), 15772.
3. Hoffmann, M. M., Brown, L., Campbell, M., Pechhold, K., Brate, A., He, X., ... & Schmitt, T. M. (2023). AFNT-211: A FAS-41BB–enhanced TCR-T cell therapy with stem-like properties targeting KRAS G12V-expressing solid tumors. Cytokine, 1, 1-50.







