Tumor Microenvironment Responsive Therapeutic Antibodies
The efficacy of immunotherapies for solid tumor has been heavily hampered by the hostility of tumor microenvironment (TME). TME is a complex and dynamic milieu, and tumor cells often undergo metabolic reprogramming to support their growth, survival, and proliferation within this microenvironment. While driving cancer development, TME-specific metabolite composition also contributes to the immunosuppressive nature and metabolic barriers of TME.
The specific metabolism of the TME has in recently years been leveraged to design therapeutic antibodies of precision tumor targeting
- pH-selective antibody drugs
Due to glycolysis, hypoxia, and deficient blood perfusion, acidosis is a hallmark of TME. This feature has been leveraged to design pH-selective antibodies of precision tumor targeting that remain inactive in bloodstream but become active when encountering an acidic environment in cancer.
The pH difference has also been utilized as switch of releasing therapeutic payload, as seen in the case of ADCs.
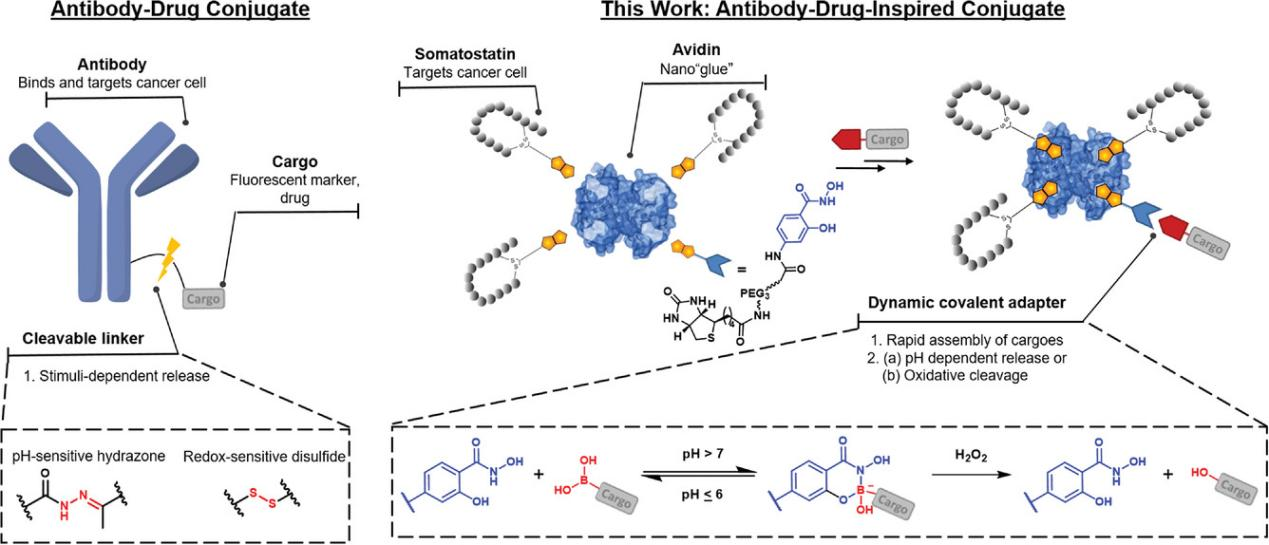
Figure 1. Designs for antibody-drug conjugate (ADC) and antibody-drug-inspired conjugates can facilitate 1) multivalent interaction with cancer cell receptors, promoting internalization. 2) Controlled cargo release is achieved through a pH-responsive or oxidative cleavage linker.
- proteolysis-responsive antibody drugs
Dysregulated (specific or elevated) proteolysis in TME has become recognized as another important hallmark of cancers.
Exploiting the unique TME characteristic, scientists have designed protease-activated T cell engager antibodies to fulfill site-specific and controlled drug delivery. Their activity is sealed with masking peptides fused to antibodies with tumor protease-cleavable linkers, and recovered by the removal of the mask in TME.
This class of antibodies are under investigation by Janux, CytomX, Roche, Amunix, Harpoon, and quite a few more.
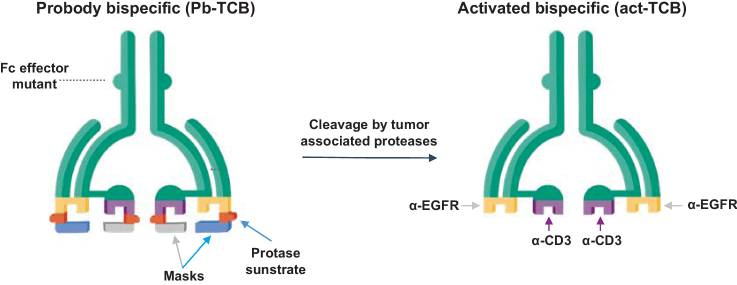
Figure 2. Probody TCB (CI107) is a bispecific IgG designed for EGFR and CD3 binding, with a cleavable linker preventing normal tissue interaction. In the tumor microenvironment, linker cleavage enables specific binding to the target antigen and CD3+ T cells.
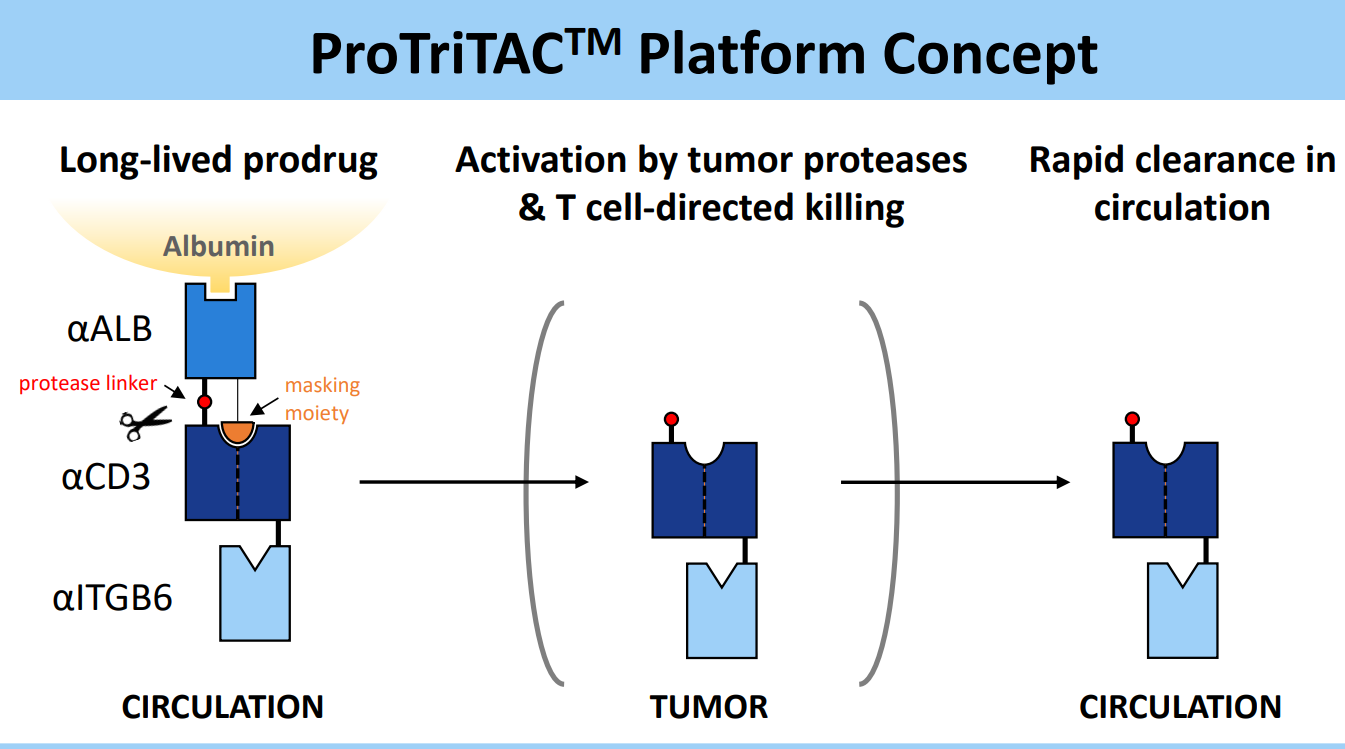
Figure 3. ProTriTACTM developed by Harpoon Therapeutics
Besides, Revitope's PrecisionGATE and Takeda's COBRA, platforms for the design of precision immunotherapies with separated, inactive constructs, leverage the proteolysis characteristic for better controlled immune activation. The constructs, upon proteolysis in TME, rearrange and become complete, active T cell engagers:
The disease specific protease-mediated cleavage, meanwhile, is also exploited in PAC-Ig platform of Chugai, where TME-targeted VHH release is designed to achieve site-specific antigen binding:
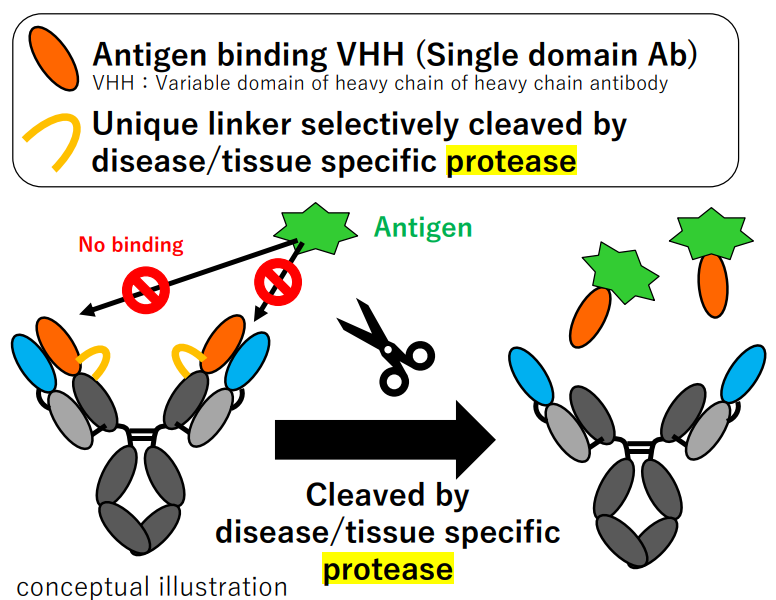
Figure 4. PAC-IgTM platform developed by Chugai
Last but not least, this TME-responsive mechanism is also utilized to deliver viral antigens to tumor surface for presentation, which in turn retargets specific CD8+ T cells against tumors:
TME-responsive precise therapeutic antibodies are an exciting and rapidly evolving area of cancer research. They hold great promise for improving the precision and effectiveness of cancer treatments, and are offering a promising avenue for more precise and personalized medicine in the future that is likely to produce better outcomes for cancer patients.
Reference
1. Sulea, Traian, et al. "Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment." MAbs. Vol. 12. No. 1. Taylor & Francis, 2020.
2. Chang, Hwai Wen, et al. "Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches." Proceedings of the National Academy of Sciences 118.9 (2021): e2020606118.
3. Lee, Peter S., et al. "Improved therapeutic index of an acidic pH-selective antibody." MAbs. Vol. 14. No. 1. Taylor & Francis, 2022.
4.Raabe, Marco, et al. "Assembly of pH‐Responsive Antibody‐Drug‐Inspired Conjugates." Macromolecular Bioscience 22.2 (2022): 2100299.
5.Migliorini, Francesca, et al. "A pH-responsive crosslinker platform for antibody-drug conjugate (ADC) targeting delivery." Chemical Communications 58.75 (2022): 10532-10535.
6.Boustany, Leila M., et al. "A Probody T Cell–engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity." Cancer Research 82.22 (2022): 4288-4298.
7.Geiger, Martina, et al. "Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody." Nature communications 11.1 (2020): 3196.
8.Cattaruzza, Fiore, et al. "Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors." Nature Cancer 4.4 (2023): 485-501.
9.Lin, Regina J., et al. "ITGB6 ProTriTAC™, a protease-activated T cell engager prodrug targeting Integrin-β6 for the treatment of solid tumors." Cancer Research 83.7_Supplement (2023): 2927-2927.
10.Panchal, Anand, et al. "COBRA™: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors." MAbs. Vol. 12. No. 1. Taylor & Francis, 2020.
11.Millar, David G., et al. "Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy." Nature biotechnology 38.4 (2020): 420-425.
Understanding the specific metabolic characteristics of TME is essential for developing targeted therapies that exploit metabolic vulnerabilities in cancer cells while potentially sparing normal cells. At DetaiBio, we understand how essential it is to tailor antibody discovery and drug design strategy according to target product profile, and, with our cutting-edge single B antibody discovery approach, are dedicated to finding antibody candidates of desired properties to meet challenging but urgent medical needs.







