Applications of Nanobodies
Since their discovery, nanobodies have been widely used in many medical research fields such as biopharmaceutical industry, IVD, oncology and immunology research. Nanobodies not only have a potential role in in vivo imaging, but their development as biologic drugs for the treatment of lung diseases, systemic lupus erythematosus, etc. has also seen great development.
Tumor Diagnosis and Definition of Cancer Biomarkers
Nanobodies can effectively penetrate in tumor tissues and can be used as good tracers to assist in early diagnosis and prevention of cancer by detecting or defining biomarkers. Compared to other diagnostic technologies based on monoclonal antibodies, the high stability of nanobodies under extreme temperature, pH or ionic strength ensures their application under harsh conditions. In addition, nanobodies offer higher detection sensitivity and more accurate results.
An ingenious and rapid strategy for generating nanobodies against unknown cancer biomarkers is through immunization of patient samples, by which researchers identified a new breast cancer-specific biomarker, cytokeratin 19 fragment (CYFRA21-1). Similarly, nanobody-based reverse proteomics technology was used for the diagnosis and treatment of glioblastoma multiforme (GBM). Mass spectrometry analysis of nanobody-antigen binding pairs allowed the identification of novel GBM biomarkers TRIM28 and β-actin.
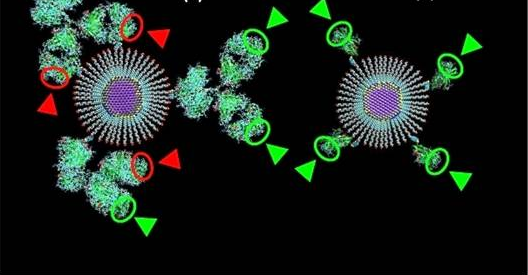
Figure 1. Highly sensitive quantum dot and nanobody coupling product detects HER2, a tumor biomarker for lung and breast cancer
Mediated Drug Delivery and Tumor Targeting Therapy
Nanobodies have a small molecular weight and are able to cross the human blood-brain barrier to the brain, where they can mediate the delivery of drugs, peptides, and other large molecules for therapeutic purposes.
Nanobodies are not affected by the reducing cytoplasmic environment and can be used as in vivo antibodies. On the one hand, some types of nanobodies help to achieve high-resolution protein analysis and better understanding of the relationship between protein structure and function. For example, a nanobody against the SH3 structural domain of the actin branching regulator dermatoglycan suggests that the SH3 structural domain function of dermatoglycan underlies endogenous phase formation and activity. It shows that nanobodies can aid in the discovery or rational design of new therapeutic agents through medicinal chemistry by enabling high-resolution protein analysis. In addition, displacing an overexpressed protein from a cell in molecular studies is relatively easy to achieve, but less so for resident endogenous proteins. However, nanobody has been labeled with a targeting sequence that allows it and the endogenous antigen to be transferred to any compartment or region within the cell, and even if the nanobody fails to bind specifically to the antigen, researchers are able to study the correlation between protein location and function and follow it in real time.
On the other hand, from a therapeutic perspective, one nanobody against CapG, a relatively unknown cytoskeletal protein, substantially curbed breast cancer metastasis in an orthotopic in vivo xenograft model. It suggests that there are many drug targets other than GPCRs or kinases/phosphatases, and by inhibiting these targets the metastatic effects of cancer cells can be diminished, and the future of nanobody applications is bright.
Nanobodies with small molecular weights that target tumor-specific receptors can be used as carriers to deliver toxins or drugs to tumors, thereby reducing non-specific toxicity to normal cells and decreasing side effects. Nanobodies lacking Fc tails also do not trigger Fc-related immunogenic responses when mediating drug transport to tumor cell receptors. Carriers generally used for nanoparticle drugs include liposomes, micelles, albumin-based nucleic acid nanoparticles (NANAPs) and polymer-based aggregates or polymers, which are widely used and effective. Due to the abundance of protein in serum, NANAPs are more biocompatible and safe. Encapsulation of multi-kinase inhibitor 17,864 in EGa1-coated NANAPs allows the lysosomes to digest the NANAPs particles through cytocytosis, allowing the inhibitor to be released in cytoplasmic solution and inhibit the proliferation of cancer cells. In addition, nanobody-mediated artificial aggregates and polymorphs composed of PEGylated PEI conjugates modified with anti-MUC1 nanobodies have also received considerable attention in recent years for targeted therapies.
Nanobody-targeted radionuclide therapy is one of the most widely used modalities to treat cancer. VHH with radiolabel identifies and binds the primary tumor site to the diseased tissue, directly increasing the radiation dose of radionuclide targeted to the tumor, which can significantly reduce the impact and damage to the surrounding normal tissues. Another way to treat cancer is photodynamic therapy, which produces fewer side effects. It is a precise and effective treatment by combining photosensitizers with nano-antibodies to target tumor tissues, and by activating the photosensitizers to trigger a photochemical reaction to produce a cytotoxic effect that kills tumor cells.
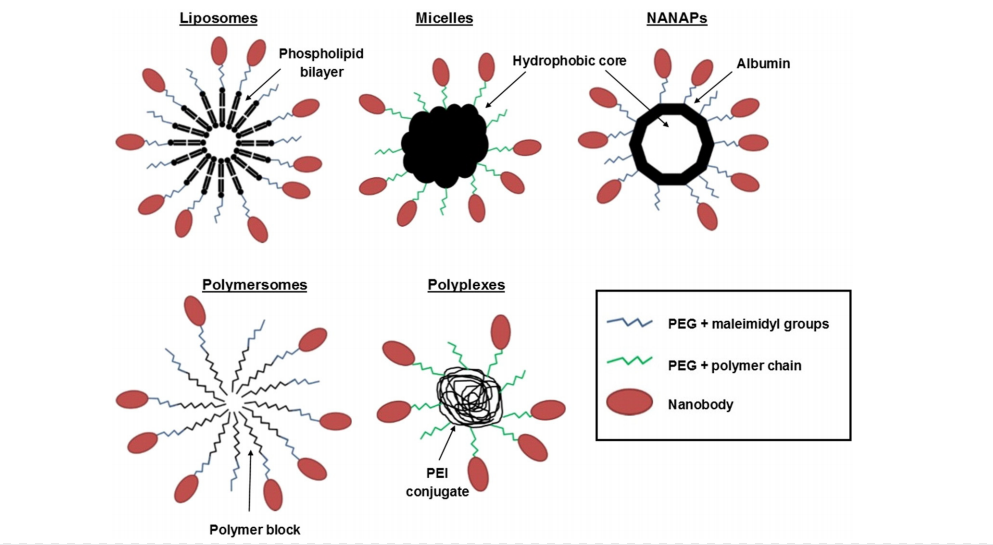
Figure2. Carriers generally used for nanoparticle drugs
Molecular Imaging
In the field of molecular imaging, small molecular weight nanobodies are very advantageous. They enable rapid aggregation and uniform distribution of tumors and are rapidly cleared in the blood, helping to improve the tumor-to-background ratio. In addition, nanobodies can easily bind to several imaging agents and possess relatively safe and high specificity.
Nanobodies are mainly used in molecular imaging for tumor-specific visualization, detection of atherosclerotic plaques, efficacy assessment and individualized therapy. The main modalities for visualization of cancer include single photon emission computed tomography (SPECT), positron emission tomography (PET), ultrasound imaging and optical tumor imaging.
In studying the correlation between labeled nanobodies and the load during tumor treatment, molecular imaging can visualize changes at the molecular level in disease treatment, which facilitates the assessment of tumor efficacy in favor of subsequent treatment.
Research and Treatment of Infectious Diseases
Nanobodies are not only suitable for the diagnosis and treatment of oncological diseases, but also have considerable potential advantages for some infection such as viruses, bacteria, parasites and toxins. Unlike conventional antibodies that cannot protect against viruses that have already invaded host cells, nanobodies not only antagonize viruses at an early stage, but can also be designed as intracellular antibodies to inhibit the replication, assembly and release of viruses. Nanobodies can also be developed for bacterial infections that are primarily treated with antibiotics, avoiding to some extent the problems associated with antibiotic resistance.
For parasites and fungi, nanobodies are currently focused on the treatment of African trypanosomiasis, or sleeping sickness. Thanks to their low molecular weight and high affinity, nanobodies are able to bind to conserved antigenic epitopes hidden by variant glycoproteins on the trypanosome surface membrane, thus interfering with trypanosome endocytosis and exhibiting direct trypanosome lysis.
Due to their antifungal effect, nanobodies are also quite widely used in anti-dandruff shampoos. In addition to this, the use of antibody drugs for antagonizing animal toxins such as snake venom toxins and bacterial toxins has been used and is expanding.
Relative Services
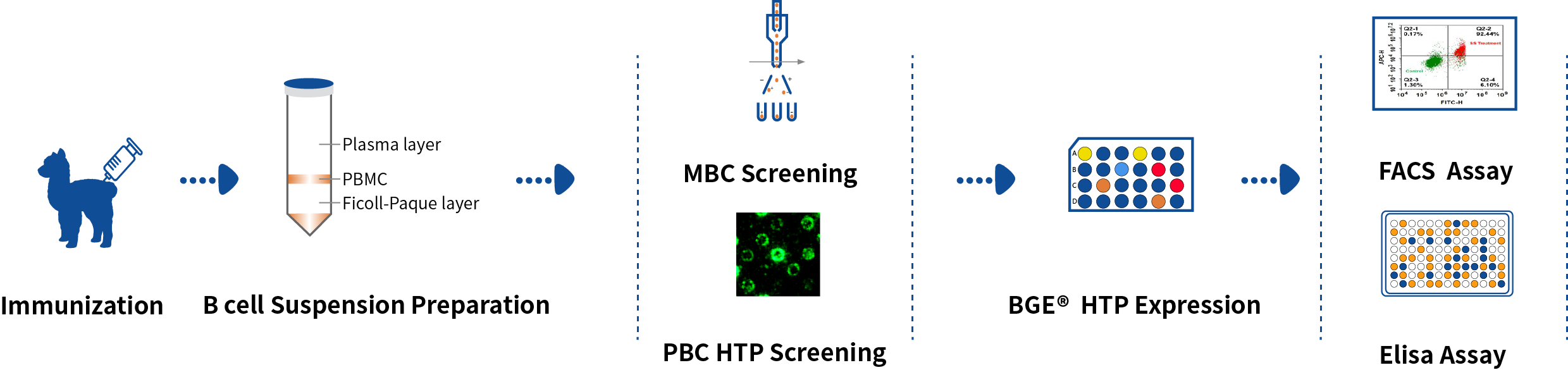
DetaiBio offers SingleB® Alpaca VHH/Nanobody Discovery Service, directly screening B cells. Dual pathway sorting of MBC and PBC to accelerate your nano-antibody discovery, guarneteeing nanobody diversity and affinity, with one-stop service from antigen preparation to antibody expression.
Reference
[1]Isabel Van Audenhove, Jan Gettemans. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer[J]. EBioMedicine,2016,8(C).
[2]D'Huyvetter Matthias, Xavier Catarina,Caveliers Vicky, Lahoutte Tony,Muyldermans Serge, Devoogdt Nick. Radiolabeled nanobodies as theragnostic tools in targeted radionuclide therapy of cancer.[J]. Expert opinion on drug delivery,2014,11(12).






