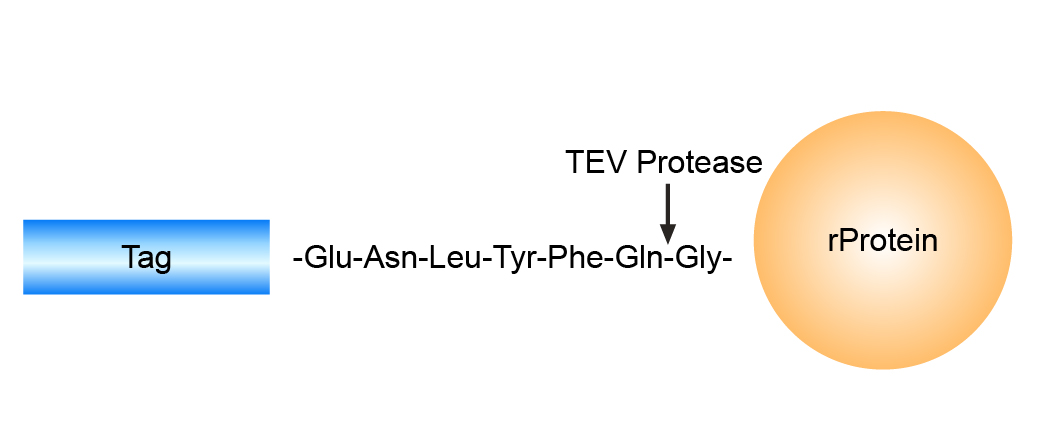
TEV Protease is a highly specific protease commonly used to remove affinity tags from fusion proteins. It precisely recognizes the heptapeptide sequence EXXYXQ↓(G/S) and cleaves between the glutamine and glycine/serine residue. TEV Protease can remove the tag while leaving only a single Gly or Ser residue at the N-terminus of the protein of interest. This minimizes structural or functional interference from residual sequences.
TEV Protease exhibits optimal activity at pH 7.0 and 30°C, while maintaining robust enzymatic performance across a broad pH range (6.0–8.5) and temperatures (4°C-30°C). It allows for seamless adaptation to the stability requirements of various target proteins during cleavage reactions.
DetaiBio's TEV Protease is highly purified recombinant protease expressed in E. coli and engineered with an N-terminal His-tag to enable efficient removal via Ni-NTA affinity purification.
Applications
Cleavage of fusion tags from recombinant proteins.
Unit Definition
One unit of TEV Protease is defined as the amount of enzyme required to cleave more than 85% of 3 μg substrate in 1 hour at 30°C, in 1× TEV Protease Buffer (50 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, 1 mM DTT).
Storage
- Store at –80°C for up to 2 years.
- Stable at –20°C for up to 6 months.
- Avoid repeated freeze-thaw cycles.
Notes
- Optimal Conditions: TEV Protease is most active at 30 °C and pH 7.0, but can be used at 4 °C for overnight cleavage to preserve target protein structure.
- Imidazole Compatibility: Maintains activity in up to 400 mM imidazole, allowing direct cleavage post–Ni-NTA purification or after dialysis.
- Inhibitor Sensitivity: Unaffected by common serine protease inhibitors (e.g., PMSF, AEBSF), but inhibited by cysteine protease inhibitors (e.g., NEM, IAA) due to its essential Cys151 residue.
- For research use only.





