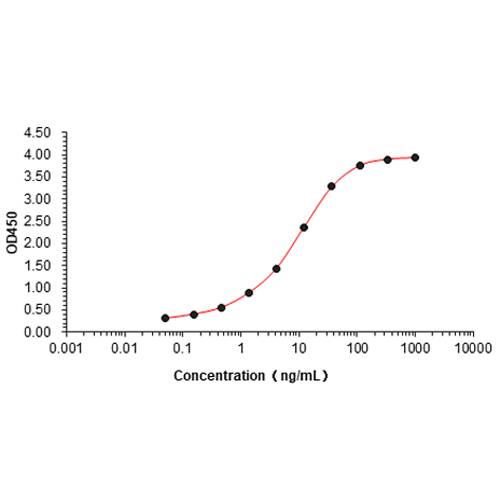
Diphtheria toxin Protein
| Product Name | Diphtheria toxin Protein |
| Product Code | DTD01
|
| Product Specifications | 0.2mg, 0.5mg, 1 mg, 5mg |
| Price | Please inquire for pricing |
Product Details
| Purity | ≥ 95% by SDS-PAGE |
| Expression Host | E.coli |
| Sequence | MGADDVVDSS KSFVMENFSS YHGTKPGYVD SIQKGIQKPK SGTQGNYDDD WKGFYSTDNK HDAAGYSVDN ENPLSGKAGG VVKVTYPGLT KVLALKVDNA ETIKKELGLS LTEPLMEQVG TEEFIKRFGD GASRVVLSLP FAEGSSSVEY INNWEQAKAL SVELEINFET RGKRGQDAKY EYMAQACAGN RVRRSVGSSL SCINLDWDVI RDKTKTKIES LKEHGPIKNK MSESPNKTVS EEKAKQYLEE FHQTALEHPE LSELKTVTGT NPVFAGANYA AWAVNVAQVI DSETADNLEK TTAALSILPG IGSVMGIADG AVHHNTEEIV AQSIALSSLM VAQAIPLVGE LVDIGFAAYN FVESIINLFQ VVHNSYNRPA YSPGHKTQPF LHHHHHH |
| Molecular Weight | 43 kDa |
| Tag | C-His |
| Endotoxin Level | <1 EU/μg |
| Buffer | 1×PBS, pH 7.4 |
Stability &
Storage Conditions | Freeze-dried powder can be stored at 4°C for three years. After reconstitution, it should be used within 2-4 weeks and can be stored at -20°C for up to 3 years. |
| SDS-PAGE | 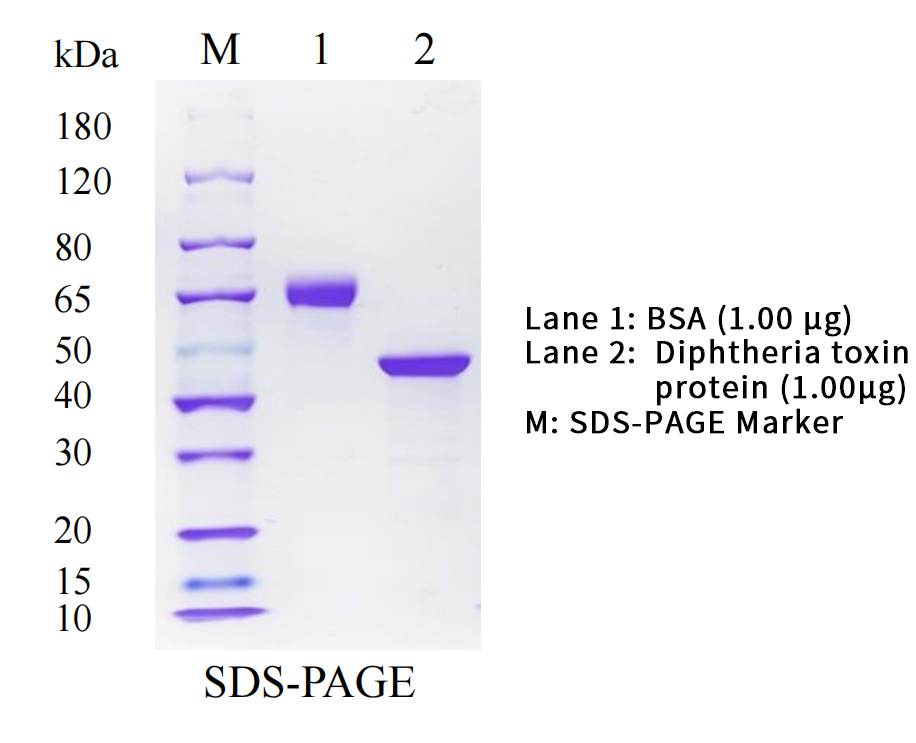 |
| Western Blot | 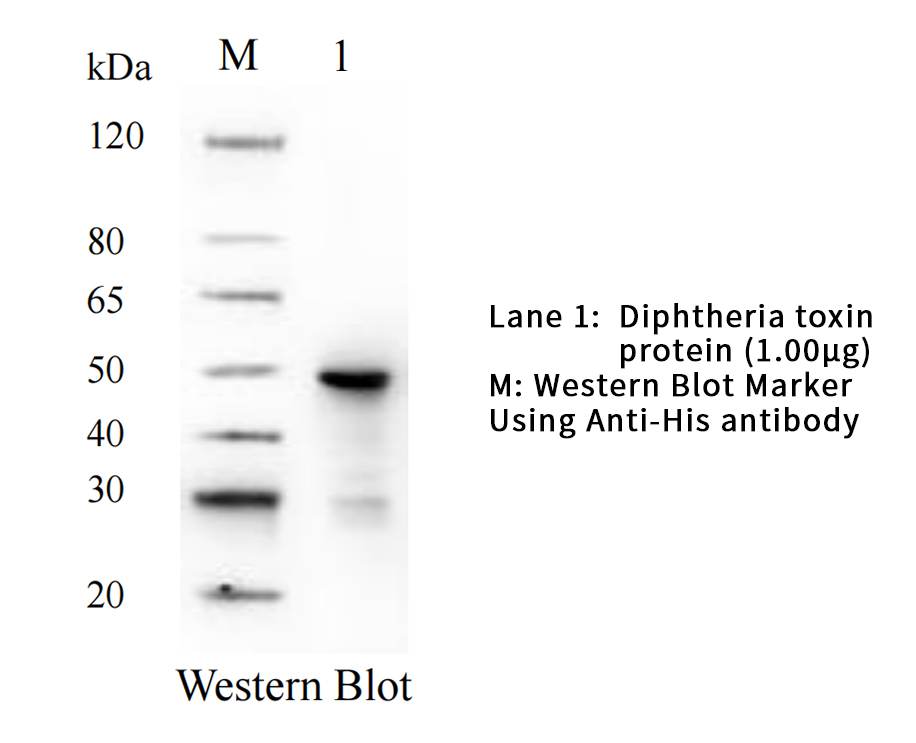 |
Diphtheria toxin is an exotoxin released by Corynebacterium diphtheriae, which has toxic molecules and is the main pathogenic substance causing diphtheria disease.
There are three structural regions: the N-terminus is the catalytic region, the middle is the transmembrane region, and the C-terminus is the receptor binding region. Under the action of trypsin, diphtheria toxin is degraded into A fragment and B fragment. DTA is the enzyme active region of diphtheria toxin and a key structural domain of DT type immunotoxins, which inhibits protein synthesis by catalyzing ADP ribosylation on chain elongation factor 2. However, Escherichia coli chain elongation factor G is not affected by this, so some diphtheria expression plasmids based on DTA fragments can be amplified in engineered bacteria. DTA protein and its monoclonal antibodies have important value in the study of the toxicity mechanism, detection, and purification of immunotoxins. At the same time, by utilizing its characteristic of producing specific immunity, it can be used to make targeted drugs. In early anti infection treatment, toxoid vaccines made from diphtheria toxin can be used for the prevention of the disease.
Rapid Mouse mAb IgG Isotyping kit
| Product Name | Rapid Mouse mAb IgG Isotyping kit |
| Product Code | DTT03
|
| Available Sizes | 10 strips (15ml preservation solution) |
| Price | Please inquire for pricing |
Product Description
Detaibio’s mouse IgG antibody subtype identification kit uses lateral chromatography immunoassay technology to quickly detect cell culture supernatant, ascites or purified mouse IgG antibody product subtypes. When testing, you only need to draw a small amount of sample and add it to the sample addition area of the test paper card, wait for 10 minutes, and then the test results can be read.
This product is for scientific research use only.
Product Overview
| Product Name | Rapid Mouse mAb IgG Isotyping Kit |
| Purpose | Detection of cell culture supernatant, ascites fluid or purified mouse IgG antibody product subtypes |
| Technology | Lateral flow immunoassay |
| Sensitivity | 20 ng/mL |
| Storage | 4-30°C, avoid direct sunlight, do not freeze |
| Validity | 12 months |
Product Contents (10 tests)
| Rapid Mouse mAb IgG Isotyping Test Strips | 10 tests |
| Sample Dilution Buffer | 15 mL |
Sample Requirement
①This reagent is suitable for detecting mouse IgG antibody products in cell culture supernatants, ascitic fluid, or purified antibodies. If the sample is visibly turbid, centrifuge and use the supernatant for testing
②Before testing, refrigerated samples should be brought to room temperature, and frozen samples should be fully thawed, mixed well, and then brought to room temperature before testing.
Test Method
①Sample dilution: (Please dilute according to the following ratios based on sample type)
1)Cell culture supernatant: 1:50 (sample: diluent), i.e., add 2 μL sample to 100 μL diluent.
2)Ascitic fluid: 1:1000 (sample: diluent), i.e., add 2 μL sample to 2 mL diluent.
3)Purified antibody: Dilute to 500 ng/mL using diluent.
4)If the antibody concentration in the sample is below 1 μg/mL, no dilution is needed; the sample can be added directly for testing.
*The above dilution methods are recommended for first-time testing. Adjustments can be made based on the sample concentration.
②Remove the test strip and place it flat on the surface, allowing it to equilibrate to room temperature.
③Take 80 μL of the sample and add it to the sample area on the reagent card, ensuring no visible air bubbles. Start the timer and read the result after 10 minutes.
Test Results
①Negative result: A red band appears at the control line (C line), and no red bands appear at the test lines (T1/T2/T3/T4/T5).
②Positive result: A red band appears at the control line (C line), and one or more red bands appear at the test lines (T1/T2/T3/T4/T5).
③Invalid result: No red band appears at the control line (C line). Retest the sample.
Q: What are the advantages of using an immunochromatographic strip for mouse IgG antibody subclass identification?
A: Currently, the determination of monoclonal antibody subclasses mainly relies on ELISA, which takes 4-6 hours per test, requiring significant time and effort. This severely delays the selection and preservation of monoclonal antibody purification methods, increasing the time and workload in the monoclonal antibody preparation process. This product is a rapid method for identifying the subclass of mouse monoclonal antibodies in cell culture supernatants or purified monoclonal antibodies using an immunochromatographic strip. It is convenient, quick, cost-effective, has high accuracy, good repeatability, and short detection time, solving the issues associated with ELISA testing.
Q: What are the sample requirements before using the mouse IgG antibody subclass identification kit?
A: Before testing, refrigerated samples should be brought to room temperature. Frozen samples must be fully thawed, mixed well, and brought to room temperature before testing. If the sample is visibly turbid, centrifuge and use the supernatant for testing.
Q: Why do the test line T color intensities vary after testing different samples?
A: When the sample does not contain His-tagged proteins, the test line T shows the darkest color. As the content of His-tagged protein in the sample increases, the intensity of the test line T color gradually weakens, eventually disappearing.
Q: What is the sample dilution ratio when using the mouse IgG antibody subclass identification kit?
A: Sample dilution: (Please dilute according to the following ratios based on sample type)
① Cell culture supernatant: 1:50 (sample : diluent);
② Ascitic fluid: 1:1000 (sample : diluent);
③ Purified antibody: Dilute to 500 ng/mL using diluent;
④ If the antibody concentration in the sample is below 1 μg/mL, no dilution is needed; use the sample directly for testing.
Ensure the dilution ratio follows the instructions for accurate results.
Q: What are the storage conditions and precautions when using the mouse IgG antibody subclass identification kit?
A: Store at 4-30°C, with a shelf life of 12 months. Avoid direct sunlight, store in a cool place, and do not freeze.
Q: Is a control group necessary when using the mouse IgG antibody subclass identification kit?
A: To ensure the accuracy of the results, it is recommended to set up both a negative and a positive control.
Precautions
- Use within the product's validity period and according to the operating instructions.
- Refrigerated samples should be equilibrated to room temperature before use.
Anti-DXd antibody
| Product Name | Mouse Anti-DXd Antibody, mAb |
| Target | DXd |
| Type | Monoclonal |
| Product Code | DTA0031
|
| Available Sizes | 50 μg, 100 μg, 500 μg, 1 mg |
| Price | Please inquire for pricing |
Product Details
| Concentration | 0.5 mg/mL |
| Host | Mouse |
| Immunogen | DXd conjugated to keyhole limpet haemocyanin (KLH) |
| Clone Number | 1M2F3 |
| Isotype | IgG1 |
| Purification Method | Protein A |
| Specificity | Recognizes DXd and structurally similar molecules; binds DS-8201 |
| Validated Applications | ELISA |
| Buffer | 1×PBS, pH 7.4, with 0.05% BSA, 50% glycerol, 0.03% Proclin 300 |
| Storage | Ship on ice; store at 2-8°C for 1-2 weeks or -20°C for long term; avoid freeze-thaw cycles |
Product Applications
| Applications | ELISA |
| Recommended Dilution | 0.00005-1 µg/mL |
Bioactivity ELISA

The DXd conjugated Trastuzumab ELISA assay is developed by using Mouse Anti DXd Antibody, mAb (Cat:DTA0031) and Goat Anti Mouse IgG Antibody[HRP], mAb as the capture and detection antibodies.
Mouse Anti DXd Antibody, mAb (Cat:DTA0031) binds with DS-8201.
Coating antigen: DS-8201, 0.1 µg
Mouse Anti DXd Antibody, mAb (Cat:DTA0031) dilution start from 1000 ng/mL.
DXd is a derivative of exatecan (DX8951f), a compound with increased activity and improved solubility compared to CPT, and described not to be an ABCC2 or ABCG1 substrate. DXd was used in several proprietary ADC programs, such as DS-8201a (Enhertu), U3-1402 and DS-6157a, conjugated at DAR8 and DS-1062a and DS-7300a conjugated at lower DAR (4) to limit their toxicity, that are either approved by the FDA (DS-8201a) or currently under clinical evaluation. Although the DXd payload presented lower passive membrane permeability than exatecan mesylate, it was found to be less myelotoxic and was therefore also selected for its improved safety profile.
The anti-payload antibody is a useful reagent in ADC research by quantifying and monitoring drug levels or accumulations in tumor tissue, and more. It can be used in PK, PD analysis and ELISA.
Related services
Human IgG1 FC recombinant protein
Product Details
| Aliases | IgG1 Fc Protein, Human; Ighg1 Protein, Human |
| Purity | ≥90% as determined by SDS-PAGE & HPLC |
| Expression Host | 293F Cells |
| Species | Human |
| Molecular Weight | The recombinant Human IgG1 Fc consists of 232 amino acids and has a predicted molecular mass of 26 kDa. Due to glycosylation, it appears as ~30 kDa in SDS-PAGE under reducing conditions. |
| Buffer | PBS, pH 7.4 |
| Shipping | Bulk packages of recombinant proteins are provided as frozen liquid. They are shipped with blue ice unless customers specify otherwise. |
| Stability & Storage | Samples are stable for up to twelve months from the date of receipt at -20°C to -80°C. Store under sterile conditions at -20°C to -80°C. For optimal storage, aliquoting is recommended. Avoid repeated freeze-thaw cycles. |
| SDS-PAGE | 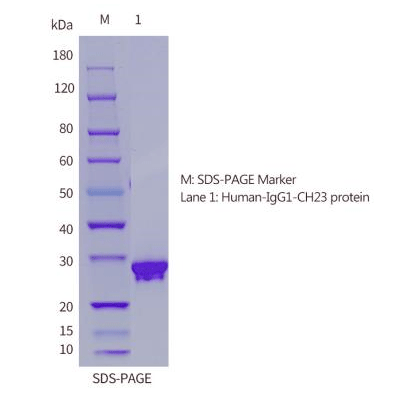 |
| HPLC | 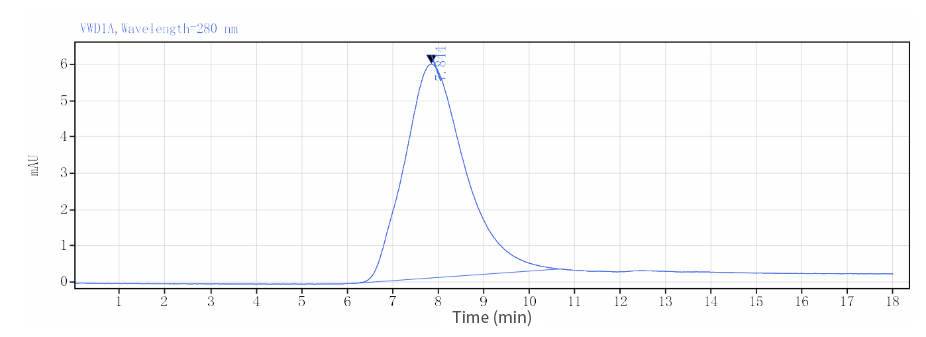 |
| ELISA | 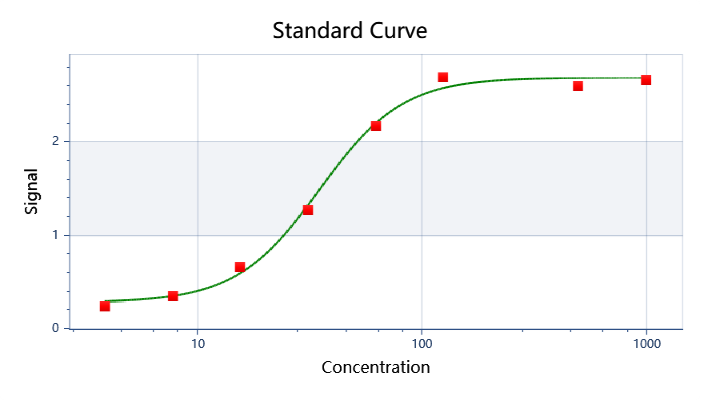 |
As a monomeric immunoglobulin that is predominately involved in the secondary antibody response and the only isotype that can pass through the human placenta, Immunoglobulin G (IgG) is synthesized and secreted by plasma B cells, and constitutes 75% of serum immunoglobulins in humans. IgG antibodies protect the body against the pathogens by agglutination and immobilization, complement activation, toxin neutralization, as well as antibody-dependent cell-mediated cytotoxicity (ADCC). IgG tetramer contains two heavy chains (5 kDa) and two light chains (25 kDa) linked by disulfide bonds, that is the two identical halves form the Y-like shape. IgG is digested by pepsin proteolysis into Fab fragment (antigen-binding fragment) and Fc fragment ("crystallizable" fragment). IgG1 is most abundant in serum among the four IgG subclasses (IgG1, 2, 3 and 4) and binds to Fc receptors (FcγR) on phagocytic cells with high affinity. Fc fragment is demonstrated to mediate phagocytosis, trigger inflammation, and target Ig to particular tissues. Protein G or Protein A on the surface of certain Staphylococcal and Streptococcal strains specifically binds with the Fc region of IgGs, and has numerous applications in biotechnology as a reagent for affinity purification. Recombinant IgG Fc Region is suggested to represent a potential anti-inflammatory drug for treatment of human autoimmune diseases.
Related products
Mouse IgG1 FC recombinant protein
Product Details
| Aliases | IgG1 Fc Protein, Mouse; Ighg1 Protein, Mouse |
| Purity | ≥90% as determined by SDS-PAGE & HPLC |
| Expression Host | 293F Cells |
| Species | Mouse |
| Molecular Weight | The recombinant Mouse IgG1 Fc consists of 232 amino acids and has a predicted molecular mass of 26 kDa. Due to glycosylation, it appears as ~30 kDa in SDS-PAGE under reducing conditions. |
| Buffer | PBS, pH 7.4 |
| Shipping | Bulk packages of recombinant proteins are provided as frozen liquid. They are shipped with blue ice unless customers specify otherwise. |
| Stability & Storage | Samples are stable for up to twelve months from the date of receipt at -20°C to -80°C. Store under sterile conditions at -20°C to -80°C. For optimal storage, aliquoting is recommended. Avoid repeated freeze-thaw cycles. |
| SDS-PAGE | 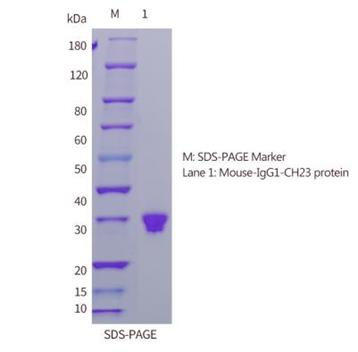 |
| HPLC | 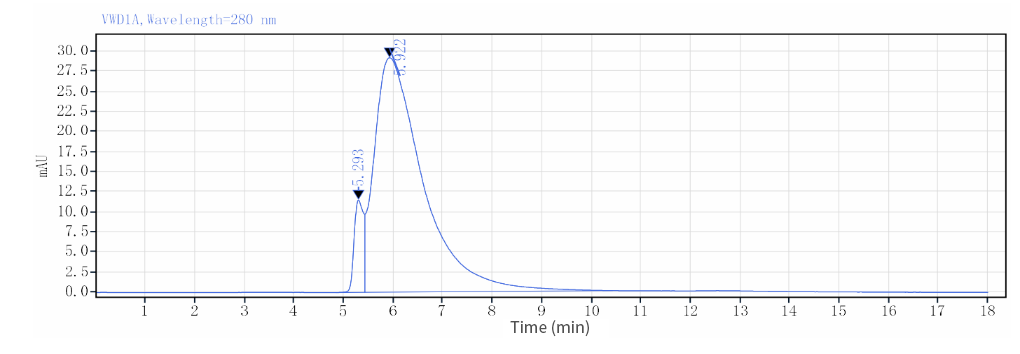 |
| ELISA | 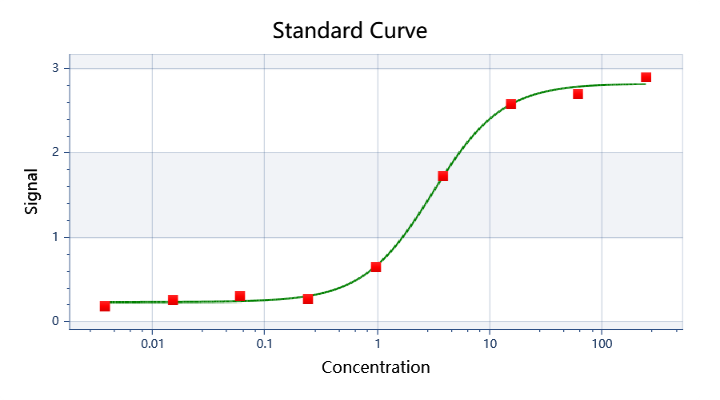 |
As a monomeric immunoglobulin that is predominately involved in the secondary antibody response and the only isotype that can pass through the human placenta, Immunoglobulin G (IgG) is synthesized and secreted by plasma B cells, and constitutes 75% of serum immunoglobulins in humans. IgG antibodies protect the body against the pathogens by agglutination and immobilization, complement activation, toxin neutralization, as well as antibody-dependent cell-mediated cytotoxicity (ADCC). IgG tetramer contains two heavy chains (5 kDa ) and two light chains (25 kDa) linked by disulfide bonds, that is the two identical halves form the Y-like shape. IgG is digested by pepsin proteolysis into Fab fragment (antigen-binding fragment) and Fc fragment ("crystallizable" fragment). IgG1 is most abundant in serum among the four IgG subclasses (IgG1, 2, 3 and 4) and binds to Fc receptors (FcγR) on phagocytic cells with high affinity. Fc fragment is demonstrated to mediate phagocytosis, trigger inflammation, and target Ig to particular tissues. Protein G or Protein A on the surface of certain Staphylococcal and Streptococcal strains specifically binds with the Fc region of IgGs, and has numerous applications in biotechnology as a reagent for affinity purification. Recombinant IgG Fc Region is suggested to represent a potential anti-inflammatory drug for treatment of human autoimmune diseases.
Related products
Mannose in Targeted Immunotherapeutic Endeavors
Mannose on the surface of many common pathogens serves as a "danger signal" that, not found on healthy human cells and tissues, activates lectin activation pathway of complement system to "bomb" pathogen invaders.
In addition to complements, macrophages are able to sense the invasion as well. When capturing mannose through receptor, the macrophage turns itself from a bored "garbage collector" to a "professional eater" or an "angry killer" against microbial attacks.
Mannose glycosylation is also found to be a “hallmark” in cancer cells.
The mannose characteristics has been evaluated for the potential in targeted and broad-spectrum microorganism inhibition and cancer diagnosis or therapy. Some of research endeavors are listed and discussed below.
- High mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting.
The aptamers tested exhibited high affinity toward spike protein of SARS-CoV-2 & envelope protein GP120 of HIV and a certain inhibition effect. Meanwhile, they specifically recognized cancer cells over normal cells.
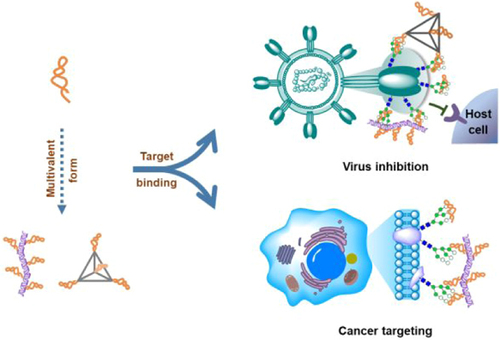
Figure 1. Mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting
- Mannose modification for targeted photothermal therapy
Photothermal therapy is promising to conquer cancer resistance. To arm it with cancer selectivity, nanoparticle used in the therapy was equipped with mannose as targeting unit. Data indicated the potential application of photothermal therapy of tumor for accurate diagnosis.
- Mannose-modification for targeting TAMs
Tumor-associated macrophages (TAMs) are a class of immune cells that amount to substantial tumor mass. They contribute significantly to immunosuppressive TME and the resistance to chemotherapy drugs and nano-medicine, and therefore are rising as targets in cancer treatment, via either polarization into M1-like phenotype or depletion.
A study with mannose-modified nanocarriers displayed TAM-targeting delivery in solid tumors.
If loaded with proper cargo, these carriers could be used for prognosis or therapeutic purposes.
Here comes the one that’s loaded with therapeutic cargo. In order to endow chemotherapy with tumor precision, a research group developed mannose-modified liposomes co-loaded with ZOL and DOX, and offered results indicating remarkably enhanced anticancer effects via depleting TAMs.
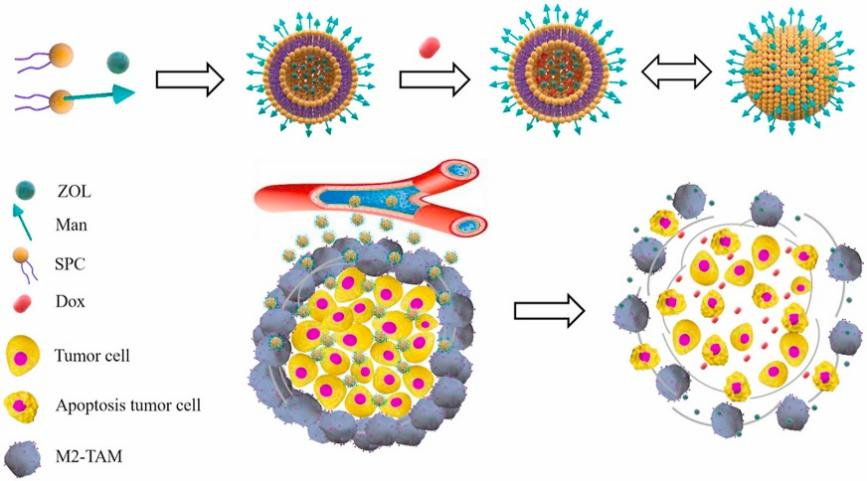
Figure 2. Man-LP@ZOL/DOX developed by Cai. could deplete M2-tumor-associated macrophages to enhance anti-tumor effect of doxorubicin on TNBC
- Lectibody that targets mannose for pan-tumor treatment.
As discussed, cancer-related glycomics, including mannose glycosylation, is found to be a “hallmark” in cancer cells, and now is a treasure waiting to be mined for pan-tumor drug development.
AvFc, a lectibody constructed by fusing glycan-binding lectin to human IgG1, was found to significantly restrict tumor growth in models, which provides POC evidence that mannose glycans in the glycocalyx of cancer cells can be a pan-tumor, druggable target.
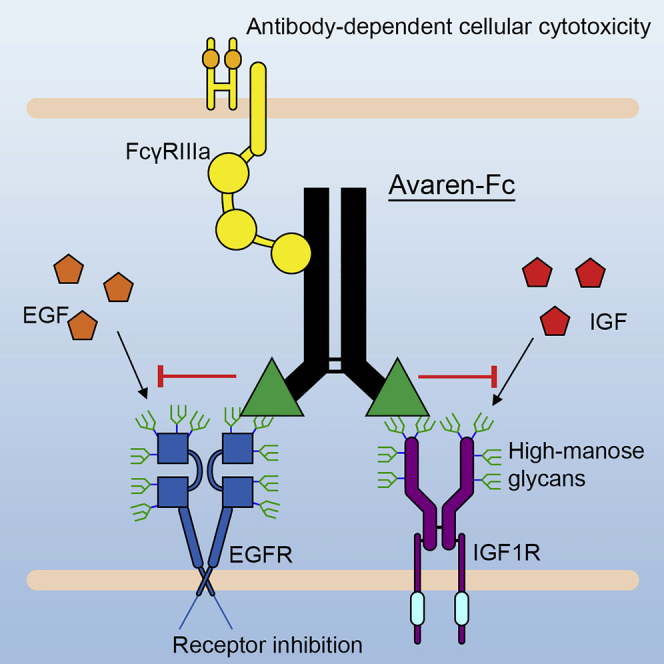
Figure 3. AvFc, a lectibody that targets mannose for pan-tumor treatment
Mannose is rarely used for glycolysis in the normal tissues of mammals, which makes it a “sweet” option in cancer treatment as in pathogen fighting, so sweet as to make “one-therapeutic-fit-all” therapies expected.
Reference
1. Bangarh, Rashmi, et al. "Aberrant protein glycosylation: Implications on diagnosis and Immunotherapy." Biotechnology Advances (2023): 108149.
2. Li, Wei, et al. "High mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting." CCS Chemistry 5.2 (2023): 497-509.
3. Li, Jiaxu, et al. "Mannose modified zwitterionic polyester-conjugated second near-infrared organic fluorophore for targeted photothermal therapy." Biomaterials Science 9.13 (2021): 4648-4661.
4. Fernández-Mariño, Iago, et al. "Mannose-modified hyaluronic acid nanocapsules for the targeting of tumor-associated macrophages." Drug Delivery and Translational Research 13.7 (2023): 1896-1911.
5. Wendong, Yao, et al. "Mannose modified co-loaded zoledronic liposomes deplete M2-tumor-associated macrophages to enhance anti-tumor effect of doxorubicin on TNBC." Journal of Drug Delivery Science and Technology 74 (2022): 103551.
6. Oh, Young Jun, et al. "Antitumor activity of a lectibody targeting cancer-associated high-mannose glycans." Molecular Therapy 30.4 (2022): 1523-1535.
With its unparalleled single B antibody discovery approach and unique antigen design strategy, Detai Bioscience, Inc. is overcoming the low immunogenicity of vitamins, toxins, and glycans, and is discovering high-quality antibodies against these haptens to enable alternative cancer therapies and upgraded in vitro diagnosis (IVD). Contact us for details of how we can help.
Tumor Microenvironment Responsive Therapeutic Antibodies
The efficacy of immunotherapies for solid tumor has been heavily hampered by the hostility of tumor microenvironment (TME). TME is a complex and dynamic milieu, and tumor cells often undergo metabolic reprogramming to support their growth, survival, and proliferation within this microenvironment. While driving cancer development, TME-specific metabolite composition also contributes to the immunosuppressive nature and metabolic barriers of TME.
The specific metabolism of the TME has in recently years been leveraged to design therapeutic antibodies of precision tumor targeting
- pH-selective antibody drugs
Due to glycolysis, hypoxia, and deficient blood perfusion, acidosis is a hallmark of TME. This feature has been leveraged to design pH-selective antibodies of precision tumor targeting that remain inactive in bloodstream but become active when encountering an acidic environment in cancer.
The pH difference has also been utilized as switch of releasing therapeutic payload, as seen in the case of ADCs.
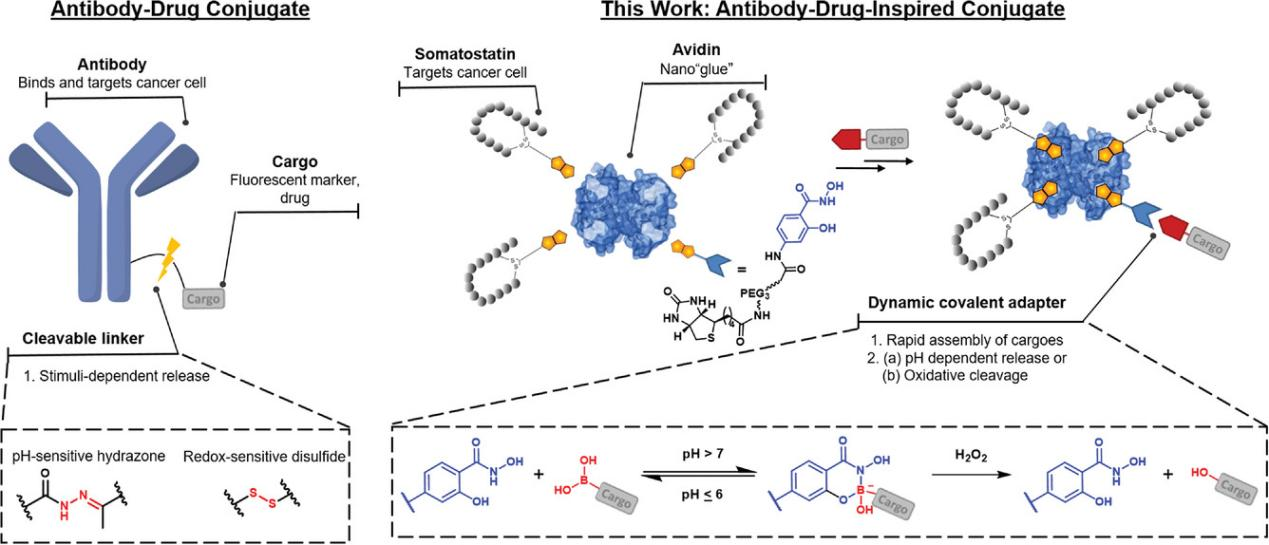
Figure 1. Designs for antibody-drug conjugate (ADC) and antibody-drug-inspired conjugates can facilitate 1) multivalent interaction with cancer cell receptors, promoting internalization. 2) Controlled cargo release is achieved through a pH-responsive or oxidative cleavage linker.
- proteolysis-responsive antibody drugs
Dysregulated (specific or elevated) proteolysis in TME has become recognized as another important hallmark of cancers.
Exploiting the unique TME characteristic, scientists have designed protease-activated T cell engager antibodies to fulfill site-specific and controlled drug delivery. Their activity is sealed with masking peptides fused to antibodies with tumor protease-cleavable linkers, and recovered by the removal of the mask in TME.
This class of antibodies are under investigation by Janux, CytomX, Roche, Amunix, Harpoon, and quite a few more.
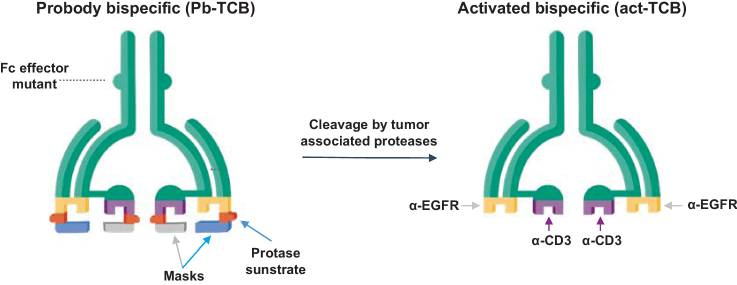
Figure 2. Probody TCB (CI107) is a bispecific IgG designed for EGFR and CD3 binding, with a cleavable linker preventing normal tissue interaction. In the tumor microenvironment, linker cleavage enables specific binding to the target antigen and CD3+ T cells.
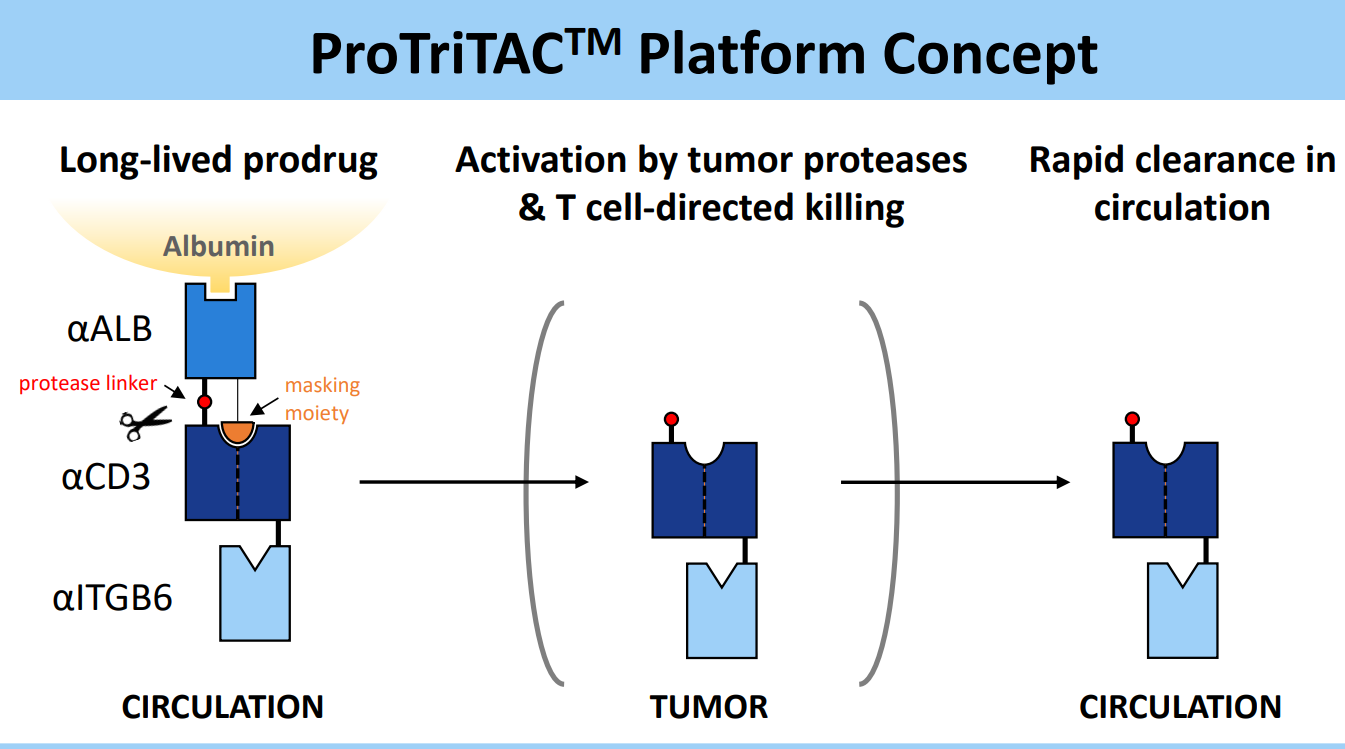
Figure 3. ProTriTACTM developed by Harpoon Therapeutics
Besides, Revitope's PrecisionGATE and Takeda's COBRA, platforms for the design of precision immunotherapies with separated, inactive constructs, leverage the proteolysis characteristic for better controlled immune activation. The constructs, upon proteolysis in TME, rearrange and become complete, active T cell engagers:
The disease specific protease-mediated cleavage, meanwhile, is also exploited in PAC-Ig platform of Chugai, where TME-targeted VHH release is designed to achieve site-specific antigen binding:
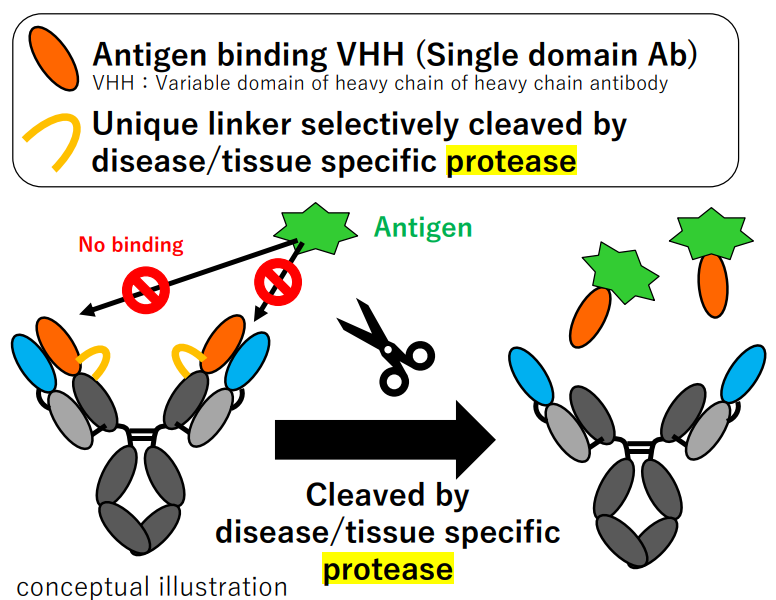
Figure 4. PAC-IgTM platform developed by Chugai
Last but not least, this TME-responsive mechanism is also utilized to deliver viral antigens to tumor surface for presentation, which in turn retargets specific CD8+ T cells against tumors:
TME-responsive precise therapeutic antibodies are an exciting and rapidly evolving area of cancer research. They hold great promise for improving the precision and effectiveness of cancer treatments, and are offering a promising avenue for more precise and personalized medicine in the future that is likely to produce better outcomes for cancer patients.
Reference
1. Sulea, Traian, et al. "Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment." MAbs. Vol. 12. No. 1. Taylor & Francis, 2020.
2. Chang, Hwai Wen, et al. "Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches." Proceedings of the National Academy of Sciences 118.9 (2021): e2020606118.
3. Lee, Peter S., et al. "Improved therapeutic index of an acidic pH-selective antibody." MAbs. Vol. 14. No. 1. Taylor & Francis, 2022.
4.Raabe, Marco, et al. "Assembly of pH‐Responsive Antibody‐Drug‐Inspired Conjugates." Macromolecular Bioscience 22.2 (2022): 2100299.
5.Migliorini, Francesca, et al. "A pH-responsive crosslinker platform for antibody-drug conjugate (ADC) targeting delivery." Chemical Communications 58.75 (2022): 10532-10535.
6.Boustany, Leila M., et al. "A Probody T Cell–engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity." Cancer Research 82.22 (2022): 4288-4298.
7.Geiger, Martina, et al. "Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody." Nature communications 11.1 (2020): 3196.
8.Cattaruzza, Fiore, et al. "Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors." Nature Cancer 4.4 (2023): 485-501.
9.Lin, Regina J., et al. "ITGB6 ProTriTAC™, a protease-activated T cell engager prodrug targeting Integrin-β6 for the treatment of solid tumors." Cancer Research 83.7_Supplement (2023): 2927-2927.
10.Panchal, Anand, et al. "COBRA™: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors." MAbs. Vol. 12. No. 1. Taylor & Francis, 2020.
11.Millar, David G., et al. "Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy." Nature biotechnology 38.4 (2020): 420-425.
Understanding the specific metabolic characteristics of TME is essential for developing targeted therapies that exploit metabolic vulnerabilities in cancer cells while potentially sparing normal cells. At DetaiBio, we understand how essential it is to tailor antibody discovery and drug design strategy according to target product profile, and, with our cutting-edge single B antibody discovery approach, are dedicated to finding antibody candidates of desired properties to meet challenging but urgent medical needs.
Fine-tuned CD3 Affinity: Desired Balance between Antitumor Activity and Safety Drives the Design of Next-generation T Cell-engagers
Cytokine release-related syndrome (CRRS) is commonly observed in T cell engagers (TCEs) clinical trials. It is triggered often by excessive T cell activation, which in turn impairs desirable drug distribution and long-term efficacy.
Is cytokine production a hallmark of T cell activation? Or, in clinical setting, are high levels of cytokine production necessary for TCE efficacy?
The answer is NO, for both questions.
Studies unveiled that activation of biological functions in CTLs is determined by the molecular dynamics between CTLs and targets, and there exists an activation THRESHOLD for cytotoxicity advancing to full activation.
These seminal findings indicated that T cell killing does NOT require the formation of a stable mature immunological synapse, and have formed rationale underlying decoupling cytotoxicity from toxicity-triggering T cell stimulation in the development of next-generation TCEs, where CD3-binding affinity is finely tuned (generally, attenuated) to reach a "sweet spot" of signal that drives anti-tumor cytolytic activity without generating excessive cytokine release and/or T-cell exhaustion.
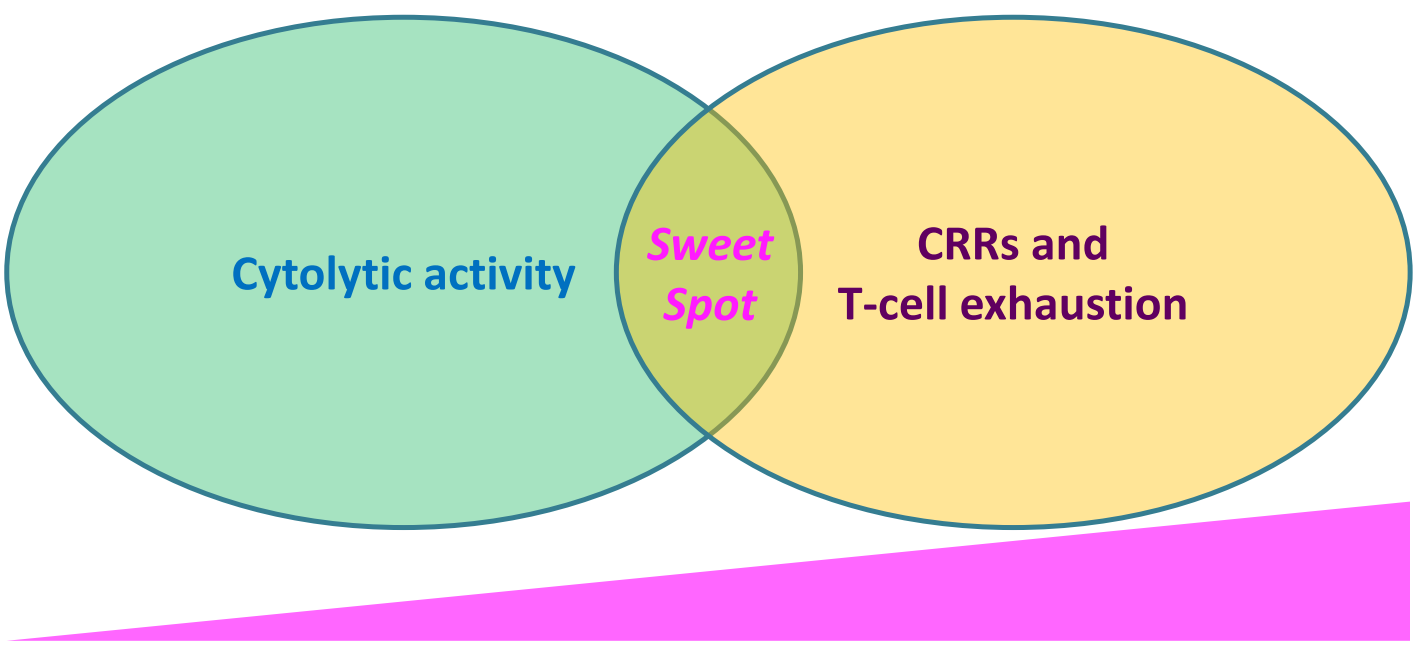
Figure 1.Fine-tuning CD3-binding affinity strikes a balance for optimal anti-tumor cytolytic activity, minimizing cytokine release and T-cell exhaustion
To date, quite a lot of novel TCEs have been built and investigated relying on the concept, and data produced so far support the "decoupling" theory, as exemplified by the developers and their candidate TCE drugs below.
- TeneoBio (TNB-486)
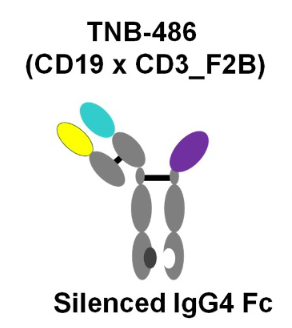
Figure 2. Structure of TNB-486
This CD19xCD3 bispecific antibody was designed for the treatment of B-NHL. Although its low affinity CD3-targeting arm lowered its in vitro killing potency, but it achieved the same maximum killing, and stimulated significantly less cytokine release. Amazingly, robust and long-term in vivo tumor cell killing occurred at low dose only. Favorable biodistribution or less propensity for inducing T-cell exhaustion or activation-induced cell death (AICD) resulting from low CD3 affinity may be responsible for its improved in vivo function.
This new TCE represented a novel therapeutic that induces potent T cell-mediated tumor-cell cytotoxicity uncoupled from high levels of cytokine release, making it an attractive candidate for B-NHL therapy.
- Genentech (HER2 TDB 1)
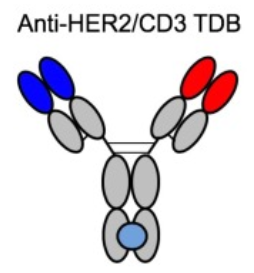
Figure 3.Structure of HER2 TDB 1
Bulit to lower systemic cytokine release and on-target/off-tumor toxicity to normal tissues of TCEs, this HER2/CD3 TCE of low binding affinity for CD3 proved that low affinity led to better overall tolerability in animal model, while T cell binding affinity had but very limited impact on in vitro and in vivo antitumor activity. The data indicated that fine-tuned affinity for CD3 as well as tumor target is a promising strategy for achieving maximal therapeutic index of CD3 TCEs.
- Amgen (AMG 424)
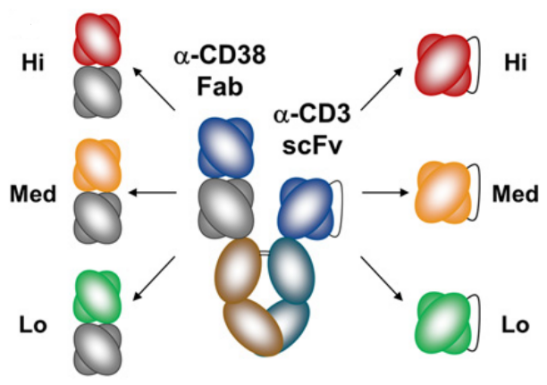
Figure 4. Structure of AMG 424
This CD3 affinity-optimized TCE showed ability to kill cancer cells and trigger T-cell proliferation, but with attenuated cytokine release, and its potential to elicit significant clinical activity in patients with multiple myeloma.
- Regeneron (REGN4018)
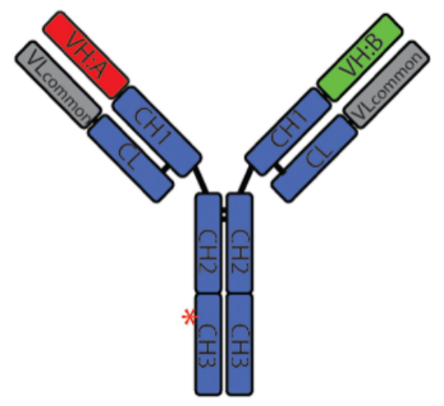
Figure 5. Structure of REGN4018
This CD3 affinity -customized MUC1xCD3 TCE for the treatment of ovarian cancer indicated attenuated profiles of cytokine release and favorable biodistribution, while its tumor killing potency remained, demonstrating potent antitumor activity and good tolerability of REGN4018, and is now under clinical evaluations.
- AbCellera
This biotech is Identifying TCEs with balanced anti-tumor potency and toxicities, mostly CRRS by fine-tuning CD3-binding affinity as well as disease target-binding moieties. Its proof-of-concept TCEs have showed high potency with low cytokine release.
Reference
1.Faroudi, Mustapha, et al. "Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold." Proceedings of the National Academy of Sciences 100.24 (2003): 14145-14150.
2.Riquelme, Erick, et al. "The duration of TCR/pMHC interactions regulates CTL effector function and tumor‐killing capacity." European journal of immunology 39.8 (2009): 2259-2269.
3.Malik-Chaudhry, Harbani K., et al. "TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL." MAbs. Vol. 13. No. 1. Taylor & Francis, 2021.
4.Staflin, Karin, et al. "Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody." JCI insight 5.7 (2020).
5.Zuch de Zafra, Christina L., et al. "Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell–recruiting antibody optimized for cytotoxicity and cytokine release." Clinical Cancer Research 25.13 (2019): 3921-3933.
6.Crawford, Alison, et al. "A Mucin 16 bispecific T cell–engaging antibody for the treatment of ovarian cancer." Science translational medicine 11.497 (2019): eaau7534.
With its cutting-edge single B antibody discovery platforms, DetaiBio is working on discovering high-quality, custom-made agonistic antibodies targeting stimulatory receptors of cytolytic effector cells for building next-generation multi-specifics, including T cell engagers and NK engagers. Visit us at www.detaibio.us to see how we can help upgrade your projects.
Molecular Construction of T Cell Engagers: Formats Matter
T cell engagers (TCEs) are artificial, multi-specific therapeutic antibodies for cancer treatment, engineered to redirect the immune system's T cells to recognize and kill cancer cells. They are designed to bind to a target antigen expressed on a cancer cell and to a trigger molecule on T cells, generally CD3. Formats of TCEs, as to other multi-specifics, matter a lot for their manufacturability and PK/ADA property as well as efficacy. Different formats have been developed, each with ads and drawbacks. Representatives of some significantly developed TCEs, either preclinically or clinically, are illustrated in this review.
IgG-like TCEs
IgG-like TCEs, due to limited mutations introduced, retain many of the favorable traits of conventional IgGs.
Unlike the generation of Catumaxomab (Epcam/CD3 TCE), specific and smart strategies to encourage desirable LC/HC and/or HC/HC pairing and to facilitate purification are essentially required and actually utilized to successfully construct the new-generation, premium format, as exemplified below.
- Mosunetuzumab by Genentech
Mosunetuzumab is constructed with knobs-into-holes technology for desired HC pairing while in vitro annealing for HC/LC pairing, and was approved for the treatment of R/R follicular lymphoma.
- Teclistamab by JNJ
It's a BCMA-targeting DuoBody that has been marketed for Relapsed/Refractory Multiple Myeloma (R/R MM) treatment
- DuoBody
A controlled Fab-arm exchange technology developed by Genmab, enables efficient generation of bsAbs with normal IgG structures.
- Epcoritamab
It's another DuoBody co-developed by AbbVie and Genmab, and was recently approved for Relapsed/Refractory diffuse large B-cell lymphoma (R/R DLBCL) treatment.
- ERY974 by Chugai (ART-Ig)
ERY974 is an anti–GPC3/CD3 TCE for treatment of solid tumors under development preclinically. Its construction is achieved through introduction of electrostatic steering mutations in the CH3 domain to drive heterodimerization of the heavy chains and utilization of a common LC to avoid LC mispairing.
- REGN1979 by Regeneron
This CD20/CD3 TCE, now in clinical trials for B-NHL, is a devised format via the introduction of local isotype chimera of IgG1/G3 in CH3 in addition to the utilization of a common LC.
This format promises to be a more native one since it avoids Fc-Fc interface engineering, therefore further reducing PK/ADA and manufacturability risks.
Based on this technology, a few more TCEs for solid tumors, including REGN4018, REGN4336(PSMA/CD3 for mCRPC), are under clinical evaluations.
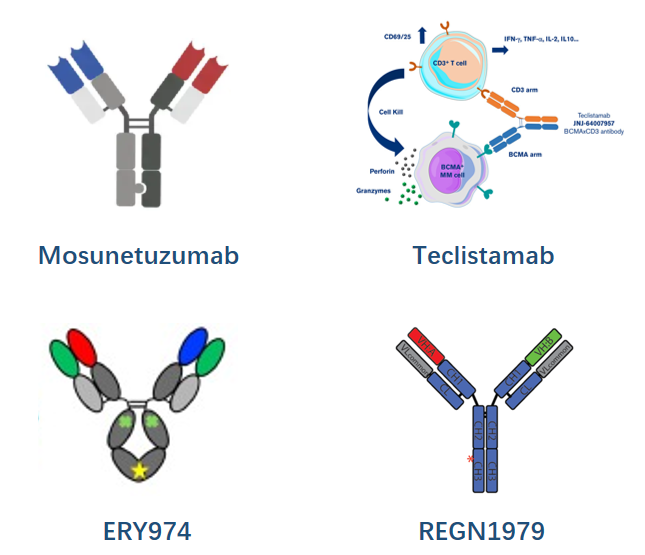
Fugure 1. Fragment-based TCEs
While IgG-like formats hold traits of native IgG favorable for manufacturability and PK/ADA property of antibody drugs, fragment-based T cell engagers (TCEs) have been developed anyway, although immunogenicity and instability result due to unnatural mutations or fragments introduced, for achieving unique medical purposes, or due to the unavailability of constructing technology, or commercial limitations.
Fragment-based TCEs are generally
- Simple structures comprised of linked scFvs, scFabs or sVDs, et al.
- Lacking half-life extending (HLE) part.
- Challenging to produce, prone to aggregate/fragment.
- Of small size with short half-life.
Representative TCE of this kind is blinatumomab(CD19/CD3 BiTE, two linked, independent scFvs) by Amgen approved for treatment of BCP-ALL and more later on.
In order to prolong half-life and allow less frequent dosing, BiTE molecules have been genetically fused to an Fc domain, resulting in a series of HLE BiTE molecules (MW around106 kD) in Amgen.
AMG757, one of HLE BiTEs by Amgen targeting DLL3, is under investigation for SCLC treatment.
In a similar fashion, MacroGenics DART platform designs therapeutic molecules that combine two independent Fabs in a diabody-like structure with or without Fc domain tailored to have a long or short half-life, respectively.
MGD024, a CD123 x CD3 Dart TCE for patients with R/R CD123(+) hematologic malignancies, has entered clinical investigation.
Meanwhile, with its TriTAC and ProTriTAC(prodrug) TCE platforms, Harpoon has created fragment-based TCEs that incorporate single domain antibodies and use albumin binding for half-life extension.
Furthermore, tebentafusp-tebn(KIMMTRAK), the first and only approved TCE for solid tumor treatment (mUM) developed by Immunocore, is composed of a TCRm and a CD3-targeting scFv. It has an acceptable PK profile due to its increased size (77KD).
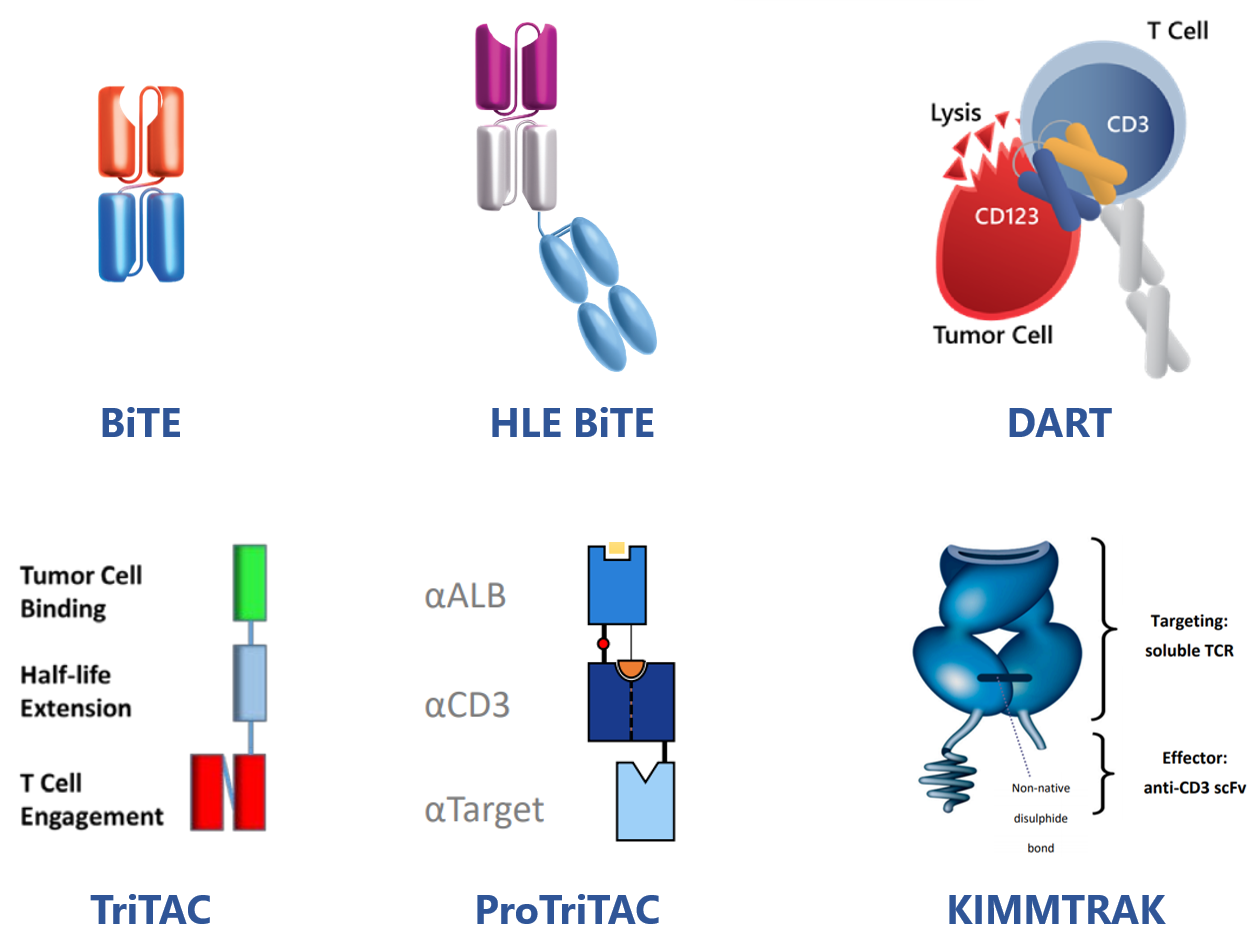
Figure 2. TCEs Other Than Above Discussed
In order to acquire improved efficacy/safety profile or to further enhance clinical significance, numerous differentiated formats in addition to fragment-based and IgG-like ones have been designed and tested, some of which are discussed below.
- EMB-07.
This TCE (ROR1/CD3) is an appended-IgG of symmetrical structure (2:2) designed with FIT-Ig platform of EpimAb for solid tumors, and is now in clinical evaluation.
Appended-IgGs are conventional IgGs appended with scFv, Fab, or nanobody, et al. They have half-life comparable to parental ones. However, non-natural structures might give rise to increased aggregation, instability and ADA risk. FIT-Ig platform generates bispecific antibodies without introducing mutations or linkers, favoring their manufacturability and stability.
EMB-06, another FIT-Ig generated TCE targeting BCMA, is also under clinical evaluation.
- BA1202.
This CEA/CD3 symmetrical (2:2) TCE by Boan Biotech is of a butterfly-shaped antibody structure: Fab ends bind bivalently to CEA while LC-NT linked bivalent scFvs bind to CD3 monovalently (possibly due to altered spatial constructure and impaired affinity compared to Fc-hinge linked one), and has entered clinical trial.
- Cibisatamab (RG7802).
It's a 2:1 format CEA/CD3 TCE by adding a second CEA-binding Fab to asymmetrical 1:1 format to enable a "low affinity but high avidity" binding to disease targets. It presumably will achieve optimized efficacy and safety by decreasing "on-target-off-tumor toxicity", and is now under development by Roche in clinical trials.
- CX-904.
Designed by Cytomx for precision targeting, this clinical-stage 2:2 EGFR/CD3 TCE prodrug is conditionally activated in tumor microenvironment upon specific proteolysis.
- Imvotamab (IGM-2323).
This TCE of IgM isotype by IGM targets CD20 and CD3. With 10-valency units for CD20, it’s proposed to bind to CD20 with more power (avidity) than IgG format, therefore overcoming target decrease-induced drug resistance, and achieving deeper depletion than currently approved antibody therapies.
More IgM TCE drugs from this company include IGM-2644 (CD38/CD3) and IGM-2537 (CD123/CD3).
- TNB-486.
This CD19-targeting TCE’s asymmetrical format by Teneobio falls kind of between the fragment-based and the IgG-like. It’s comprised of a CD3-binding half IgG and a CD19-binding HcAb, and is now under clinical evaluation for relapsed/refractory (R/R) B-lymphoma.
- YBODY.
Similar with the format of TNB-486, YBODY platform by YZYBio designs TCE that is composed of disease target-binding half IgG and CD3-binding scFv.
A few YBODY TCEs are now in clinical trials, such as M802 (HER2/CD3).
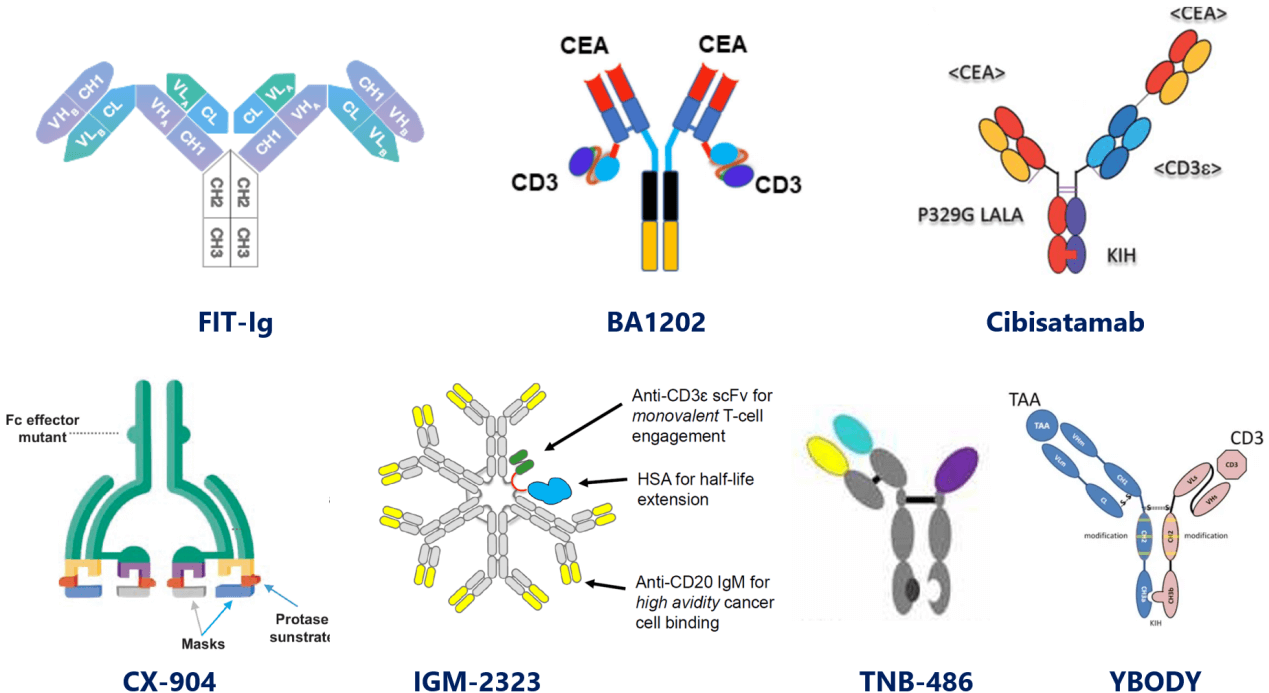
Figure3. IgG-like TCEs in differentiated formats
Reference
1.Giese, Glen, et al. "Bispecific antibody process development: Assembly and purification of knob and hole bispecific antibodies." Biotechnology progress 34.2 (2018): 397-404.
2.Pillarisetti, Kodandaram, et al. "Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma." Blood advances 4.18 (2020): 4538-4549.
3.Labrijn, Aran F., et al. "Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange." Proceedings of the National Academy of Sciences 110.13 (2013): 5145-5150.
4.Zhang, Jimin, et al. "Pharmacodynamic activity of epcoritamab (GEN3013; CD3xCD20) as monotherapy is maintained in combination with standard of care therapies in patients with diffuse large B-cell lymphoma." Cancer Research 83.7_Supplement (2023): 3248-3248.
5.Shiraiwa, Hirotake, et al. "Engineering a bispecific antibody with a common light chain: Identification and optimization of an anti-CD3 epsilon and anti-GPC3 bispecific antibody, ERY974." Methods 154 (2019): 10-20.
6.Ishiguro, Takahiro, et al. "An anti–glypican 3/CD3 bispecific T cell–redirecting antibody for treatment of solid tumors." Science translational medicine 9.410 (2017): eaal4291.
7.Smith, Eric J., et al. "A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys." Scientific reports 5.1 (2015): 17943.
8.Leung, Shuk on Annie, and Panagiotis A. Konstantinopoulos. "Advances in the treatment of platinum resistant epithelial ovarian cancer: an update on standard and experimental therapies." Expert Opinion on Investigational Drugs 30.7 (2021): 695-707.
9.Kelly, William Kevin, et al. "A phase 1/2 study of REGN4336, a PSMAxCD3 bispecific antibody, alone and in combination with cemiplimab in patients with metastatic castration-resistant prostate cancer." (2022): TPS5105-TPS5105.
10.Portell, Craig A., Candice M. Wenzell, and Anjali S. Advani. "Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia." Clinical pharmacology: advances and applications 5.sup1 (2013): 5-11.
11.Jen, Emily Y., et al. "FDA approval: blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease." Clinical Cancer Research 25.2 (2019): 473-477.
12.Giffin, Michael J., et al. "AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer." Clinical Cancer Research 27.5 (2021): 1526-1537.
13.Huang, Ling, et al. "Multispecific, Multivalent Antibody‐Based Molecules Engineered on the DART® and TRIDENTTM Platforms." Current Protocols in Immunology 129.1 (2020): e95.
14.Winer, Eric S., et al. "A Phase 1, First-in-Human, Dose-Escalation Study of MGD024, a CD123 x CD3 Bispecific Dart® Molecule, in Patients with Relapsed or Refractory CD123-Positive (+) Hematologic Malignancies." Blood 140.Supplement 1 (2022): 11753-11754.
15.Hua, Gwen, Daniel Carlson, and Jacqueline R. Starr. "Tebentafusp-tebn: A Novel Bispecific T-Cell Engager for Metastatic Uveal Melanoma." Journal of the Advanced Practitioner in Oncology 13.7 (2022): 717.
16.Bacac, Marina, et al. "A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors." Clinical Cancer Research 22.13 (2016): 3286-3297.
17.Boustany, Leila M., et al. "A Probody T Cell–engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity." Cancer Research 82.22 (2022): 4288-4298.
18.Hart, Kevin C., et al. "High valency of IGM-2323, a CD20xCD3 IgM bispecific T cell engager, displaces rituximab binding and induces potent B lymphoma cell killing." Cancer Research. Vol. 82. No. 12. 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA 19106-4404 USA: AMER ASSOC CANCER RESEARCH, 2022.
19.Malik-Chaudhry, Harbani K., et al. "TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL." MAbs. Vol. 13. No. 1. Taylor & Francis, 2021.
20.Hou, Jing-Zhou, et al. "Interim Results of the Phase 1 Study of Tnb-486, a Novel CD19xCD3 T-Cell Engager, in Patients with Relapsed/Refractory (R/R) B-NHL." Blood 140.Supplement 1 (2022): 1474-1475.
21.Jacobs, R., R. Nair, and S. G. Cho. "High complete response rate with TNB-486 in relapsed/refractory follicular lymphoma: Interim results from an ongoing Phase I study." European Hematology Association (EHA) (2023): 8-11.
22.Yu, Shengnan, et al. "A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells." Journal of Experimental & Clinical Cancer Research 38 (2019): 1-16.
DetaiBio provides high-quality design & expression services for mono- and multi-specific antibodies, including scFv, Fab, (Fab')2, VHH, chimeric antibody, bispecific antibody of variable formats, Fc fusion protein, full-length IgG and IgM. The services cover fast, small amount production, stable cell line construction and industry-level antibody production, and more. Please contact DetaiBio for more details.
Emerging Antibody Therapies Targeting B Cell Lymphomas
Since rituximab, novel modalities of highly potent immunotherapies have been investigated and/or marketed to lift the limits on treatment of B cell lymphomas, including NHLs. The past decade has witnessed the approval of plenty of antibody-based, potent therapies of B cell lymphomas, that, along with CAR -Ts, have tremendously changed the therapeutic landscape, including:
1. T Cell Engagers (TCEs)
- 1) Blinatumomab: CD19/CD3 TCE of BiTE format, by Amgen.
- 2) Mosunetuzumab: first ever approved CD20/CD3 TCE, by Roche.
- 3) Epcoritamab: a CD20/CD3 TCE by Abbvie and Genmab; subcutaneously. administrated for better patient compliance and lower CRS liabilities.
- 4) Glofitamab: a 2:1 format CD20/CD3 TCE by Roche; dual-targeting brings T cells in closer proximity for potent target-killing.
2. Antibody-drug conjugates (ADCs)
- 1) Brentuximab vedotin (anti-CD30-MMAE by Seagen/Takeda).
- 2) Polatozumab vedotin (anti-CD79b-MMAE by Genentech).
- 3) Loncastuximab tesirine (anti-CD19-PBD by ADC/Sobi).
However, the clinical effectiveness of approved ones has been impaired by inadequate treatment efficacy, intolerable toxicities, and the development of treatment resistance.
Fortunately, novel antibody therapeutics inspired by unmet medical needs are emerging that, either at early or POC stages or late stage of clinical tests, seem promising for combatting limitations currently seen in clinical practices, as exemplified below.
1. IGM-2323 (Imvotamab) by IGM Biosciences
This TCE of IgM isotype targets CD20 and CD3. With 10-valency units for CD20, it’s designed to bind to CD20 with more power (avidity). Preclinical research demonstrates that it may have advantages over IgG bispecific antibodies, therefore more likely to overcome CD20 low abundance or loss-induced drug resistance, and achieve deeper depletion than currently approved antibody therapies.
Data generated from its Phase 1 clinical trials provide evidence that Imvotamab exhibits a favorable safety and tolerability profile with encouraging activity in refractory or relapsed NHL patients.
2. Odronextamab by Regeneron
This CD20/CD3 TCE is a devised format via the introduction of local isotype chimera of IgG1/3, and promises to be of native format with improved PK property.
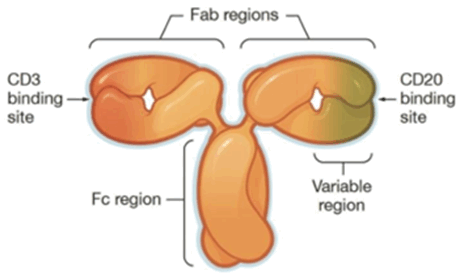
Figure 1. Structure of Odronextamab
Odronextamab has reported new data from its pivotal Phase II study, and is now under FDA priority review.
If approved, odronextamab would be the first and only bispecific antibody approved in both FL and DLBCL – the two most common subtypes of NHL.
3. GB261 (by Genor)
This CD20/CD3 TCE is of ultra-low affinity to bind CD3 and, RARELY, keeps Fc functions (ADCC and CDC). By its designed MOAs, Fc-mediated cytolysis and T cell-mediated killing dominates in according to the change of T/B cell ratios from initial stage of treatment towards late stage.
GB261 significantly inhibited rituximab-resistant cancer cell proliferation and induced but less cytokine release, and, now in clinical trial, promises to be a highly potent TCE for B cell malignancies.
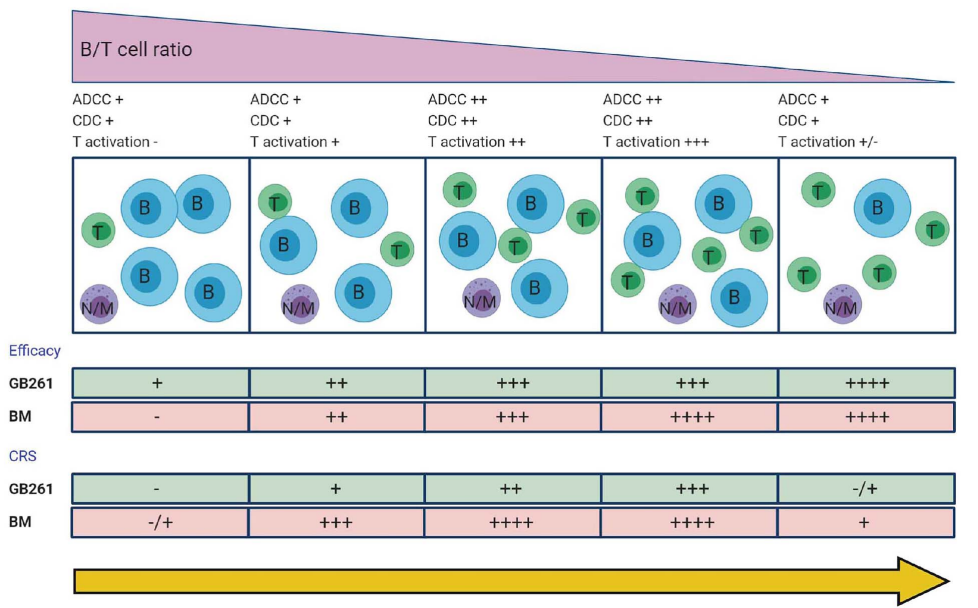
Figure 2. Efficacy of GB261 on CD20+, CD3+ lymphocytes in Cynomolgus monkeys
4. CX-2029 (by CytomX)
This probody drug conjugates directed against CD71 that contains MMAE are conditionally activated, masked ADC, whose activity would be restored by tumor-associated proteases, thereby restricting target engagement to the tumor, creating a therapeutic window for this previously undruggable target.
Now under phase 1/2 evaluation in patients with some solid tumors and DLBCL,
This prodrug showed clinical activity at tolerable doses.
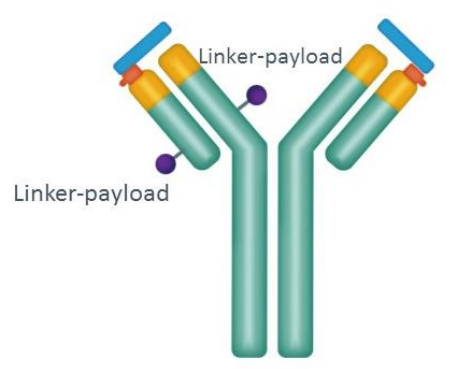
Figure 3. Structure of CX-2029 drug conjugates
5. CH2-aIgM-MMAE (by Technical University of Darmstadt)
When targeting such antigens as mentioned above, while failing to cover all target disease cells, it’s hard to distinguish cancerous B cells from their non-malignant counterparts, leading to on-target toxicities.
An amazing work on CH2-aIgM-MMAE, a human IgM-targeting prodrug ADC, provided a novel mechanism for NHL treatment.
In this excellent POC research:
- 1) Human IgM (constant domain), rather than any CD markers, was targeted for clonal B cell lymphoma eradication. Immunization of chicken instead of mammals, was used to get desired immune response.
- 2) Chicken-derived aIgM was fused to and masked by the epitope-holding IgM domain CH2 via a dual tumor-protease cleavable linker.
- 3) The prodrug was ultimately equipped with MMAE.
- 4) Binding capacity of the prodrug was restored upon conditional protease-mediated demasking which consequently enabled target-dependent antibody internalization and subsequent induction of apoptosis in malignant B cells.
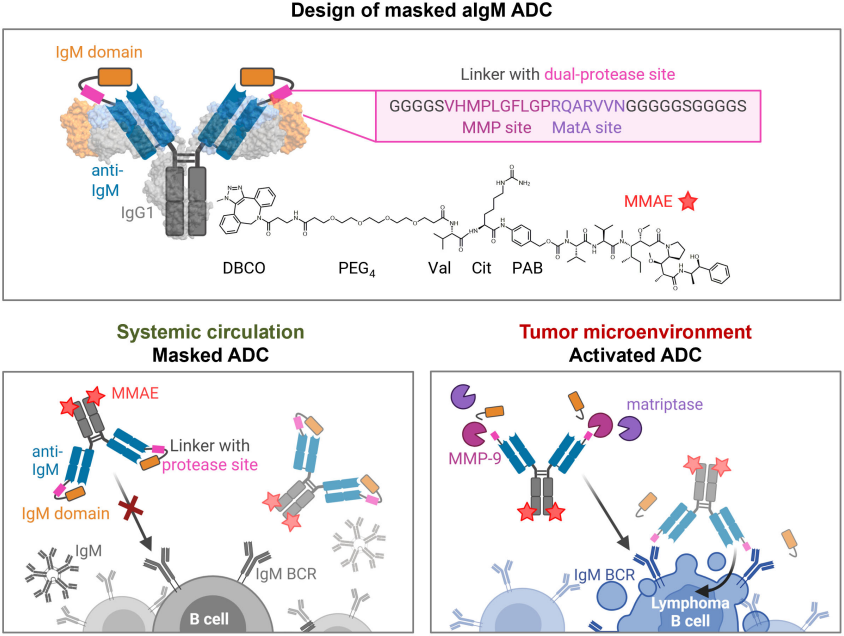
Figure 4. Design and mode of action of masked aIgM ADC.
Limitations on clinical practice are being updated as its boundary are pushed. So are the therapeutic approaches for them. As they are evolving, these approaches will definitely further enhance the chance of cure for B cell malignancies.
Reference
1. Hart, Kevin C., et al. "High valency of IGM-2323, a CD20xCD3 IgM bispecific T cell engager, displaces rituximab binding and induces potent B lymphoma cell killing." Cancer Research. Vol. 82. No. 12. 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA 19106-4404 USA: AMER ASSOC CANCER RESEARCH, 2022.
2. Cai, Wenyan, et al. "Biological activity validation of a computationally designed Rituximab/CD3 T cell engager targeting CD20+ cancers with multiple mechanisms of action." Antibody Therapeutics 4.4 (2021): 228-241.
3. Johnson, Melissa, et al. "P01. 06 CX-2029, a probody drug conjugate targeting CD71 in patients with selected tumor types: PROCLAIM-CX-2029 dose expansion phase." Journal of Thoracic Oncology 16.3 (2021): S238.
4. Johnson, Melissa, et al. "Phase I, first-in-human study of the probody therapeutic CX-2029 in adults with advanced solid tumor malignancies." Clinical Cancer Research 27.16 (2021): 4521-4530.
5. Schoenfeld, Katrin, et al. "Conditional activation of an anti-IgM antibody-drug conjugate for precise B cell lymphoma targeting." Frontiers in Immunology 14 (2023): 1258700.
Detai Bioscience, Inc. is leveraging its single B platform to unlock next-generation antibody discovery generated by rare species. We are working on cases of horse, swine, human (PBMCs), and more to come. Contact us to have rare species-generated antibody custom-made for you.