What are the main techniques currently used to obtain monoclonal antibodies?
Monoclonal antibodies (mAb) are highly specific and functionally far superior to polyclonal antibodies (pAb), and are one of the most important types of biologics in the pharmaceutical market, widely used as highly specific diagnostic tools, and therapeutic drug development.
1) Hybridoma technology
Hybridoma technology was the first technology developed for the isolation of mAb. Since its development has revolutionized the discovery of mAb. The principle of the technique is to immortalize short-lived B cells by fusion with myeloma cells to produce a monoclonal immortalized hybridoma cell line, which is then screened for antigen-specific clones in its supernatant and further subcloned for cycling to produce strict monoclonality, usually subcloned for 2 to 3 cycles.
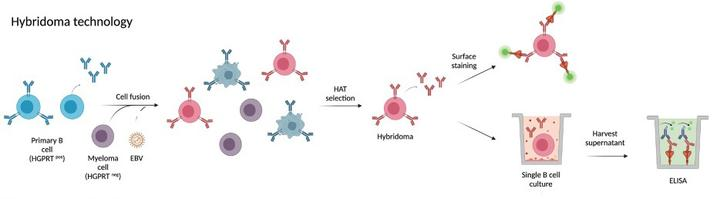
Fig.1 Process of hybridoma technology
Disadvantages:Hybridoma antibody development has a long cycle, excluding immunization, which takes approximately 4-6 months. Limited by the hybridoma fusion rate, only a small portion of B cells in the entire B cell population complete the fusion and the vast majority are lost, making it unsuitable for comprehensive screening of large antibody libraries. In addition, there is competition between different hybridomas in the same culture, and some non-secreting or low-secreting clones may exhibit faster growth rates than high-secreting clones.
2) Display technology
Methods of discovering and selecting monoclonal antibodies also include display techniques such as phage and yeast display. Currently phage display has been successfully used to screen a large number of antibody libraries. The principle of phage display technology is to insert a segment of exogenous gene into the appropriate position of the structural gene of phage shell protein. Under the condition that the reading frame is normal and does not affect the normal function of the shell protein, the exogenous gene will be expressed along with the expression of the shell protein, which results in the presentation of the polypeptide or protein on the surface of phage in the form of fusion protein
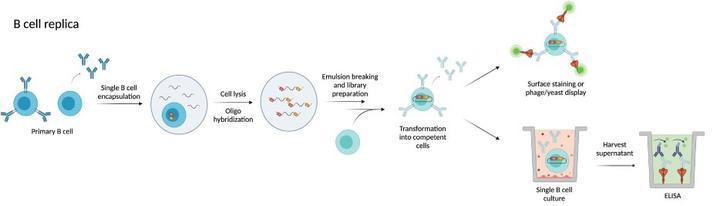
Fig.2 Process of display technology
DISADVANTAGES:It is critical to obtain natural VH-VL pairs in antibody development, however, demonstration techniques often rely on random combinations to form unnatural VH-VL antibody pairs. It has been suggested that the theoretical human B-cell library is 1012-1018, but the actual number of different B-cell clones in vivo is about 1 × 107 -2 × 107 due to sample size, out-of-frame mutations inserted during V(D)J recombination, and self-reactive BCR deletion, whereas in display technologies such as phage, the size of initial B-cell libraries is usually larger than 109, the number far exceeds that of natural B cell libraries, and the proportion of VH-VL unnatural matches is large.
3) Single B-cell Technology
Primary antigen-specific B cells are the main source of antigen-specific mAb, and single B-cell antibody technology allows for the direct isolation of single B cells from peripheral blood or lymphoid tissues in either a random or antigen-selective manner. Random B-cell isolation allows cell selection by micromanipulation, laser capture microdissection, and fluorescence-activated cell sorting (FACS). Antigen selection can be performed by screening antigen-specific B cells using antigen-coated magnetic beads, fluorescent dye-labeled antigens for multiparametric FACS, hemolytic plaque assays, and fluorescent focusing.
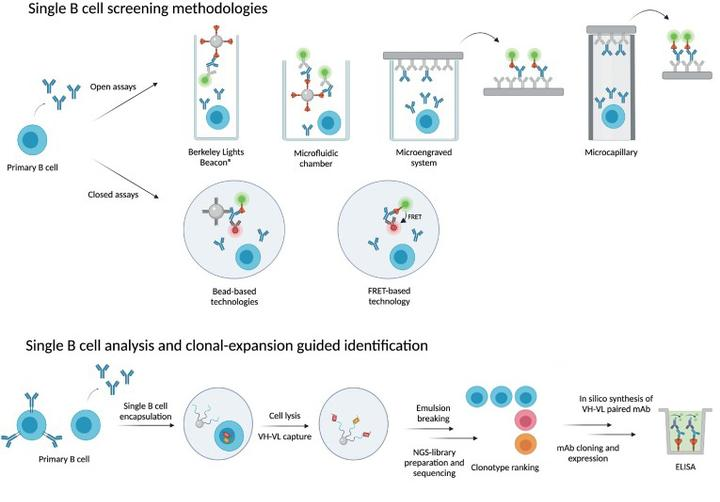
Fig3. Process of single B-cell antibody technology
Advantages of Single B-Cell Technology
The Single B-Cell Antibody Technology uses a relatively small number of cells to obtain a specific monoclonal antibody and is extremely efficient in mAb development. Single B-cell antibody technology can isolate functional mAb with conformational determinants that are difficult to mimic in vitro.FACS technology can also clearly distinguish the developmental and differentiation stages of B cells to be sorted based on the expression pattern of specific cell surface markers, and B cells at almost any stage of development can be sorted. The mAb obtained through single B cells, which are naturally matured in vivo, have a fairly high specificity for the target. In addition, the single B-cell antibody technology allows human antibodies to be obtained directly from human specimens or fully human transgenic mouse PBMCs, which is convenient and far easier and more effective than humanization via mAb from other species (e.g., mice, rats, and rabbits).
DetaiBio provides the one-stop antibody discovery service with an innovative self-developed Single B cell platform. DetaiBio SingleB® can get hits from both memory and plasma B cells of multiple species. Our in-house developed single B cell PCR kit that retrieves antibody V regions of the maximal diversity, within very short timeline-from immunization to hits: as fast as 29 days (mouse) and 49 days (rabbit). These advantages and other key features make desired antibody generation in DetaiBio happen in a fast-and-easy way. The excellent CDR diversity of obtained antibodies maximizes the possibility of getting candidate(s) robust enough to survive antibody drug development procedures that are full of risks of many kinds, or smart enough to well meet other unique or unusual application needs.
SingleB® Fast Antibody Discovery Technology
High efficiency: as fast as 29 days, saving 120 days compared to conventional hybridomas.
"1+1" Dual Screening: Memory B-cell + Plasma Cell Dual Screening with Rich Antibody Diversity.
Pre-screening:DeepLight® On-chip Cell Screening technology enables pre-screening of antibody function.
Natural maturation:the antibody is naturally matured in vivo with natural VH-LH pairing.
Comprehensive testing:Antibodies are subjected to Affinity Ranking, FACSBinding/Blocking and other multiple tests.
More choices: multiple species are available such as normal mice, rabbits, alpacas, and fully-humanized transgenic mice.
Reference
[1] Tiller T. Single B cell antibody technologies. Nat. Biotechnol. 2011, 28(5): 453-7.
[2] Marasco W.A., Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25: 1421–1434.
[3] Alessandro Pedrioli, Annette Oxenius. Single B cell technologies for monoclonal antibody discovery. Trends in Immunology. 2021, 42(12): 1143-1158.
[4] Kuppers R. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993, 12: 4955–4967.
[5] Obiakor H. A comparison of hydraulic and laser capture microdissection methods for collection of single B cells, PCR, and sequencing of antibody VDJ. Anal. Biochem. 2002, 306: 55–62.
[6] Wardemann H. Predominant autoantibody production by early human B cell precursors. Science. 2003, 301: 1374–1377.






