Rabbit Monoclonal Antibody for Detection - Higher Performance & Higher Sensitivity
Rabbit Monoclonal Antibody for Detection
Antibodies are the most commonly used tools in biological research. They are used in various assays such as Western Blot (WB), immunoprecipitation (IP), immunofluorescence (IF), immunohistochemistry (IHC) and ELISA. The two most common hosts that produce monoclonal antibodies for detection are rabbits and mice. Rabbit monoclonal antibodies for detection have the following advantages over murine monoclonal antibodies.
Antigen Recognition and Antibody Repertoire Diversity
First, rabbits, as hosts, recognize more antigenic epitopes and produce a greater variety of antibodies than mice. Rabbits have larger spleens and other immune organs than mice and can produce more lymphocytes (B cells), corresponding to more antibodies. Second, in mice, antigens such as small molecules and peptides are usually with low immunogenicity, while rabbits can also generate an immune response to these low immunogenicity antigens. Moreover, high mutations in somatic cell gene conversion and variable regions affecting immunoglobulin (Ig) genes were more common in rabbits than in rodents. This ultimately contributes to the expansion of the rabbit monoclonal antibody library. Thus, the resulting rabbit antiserum will contain a greater variety of antibodies compared to the mouse antiserum, which means there is a greater chance of finding the ideal clone for amplification.
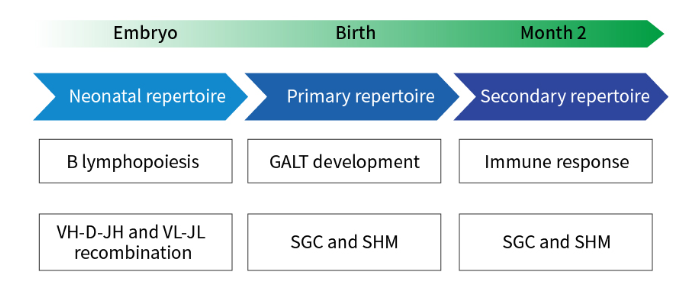
Figure 1. Rabbit B-cell and antibody library development
In addition, the Immunodominance is low in rabbits, so that antibodies produced in rabbits typically recognize more epitopes than those produced in mice. Immunodominance refers to the fact that specific epitopes are more immunogenic than other epitopes of the same antigen, which results in the immune system producing antibodies primarily against immunogenically dominant epitopes. The immune system of rabbits is less immunodominant than the immune system of mice, which allows for a broader range of detected epitopes and a higher likelihood of finding unique epitopes when developing rabbit monoclonal antibodies.
Affinity and Specificity
In general, rabbit monoclonal antibodies are similar to traditional mouse monoclonal antibodies, but have better affinity and specificity. After performing immunization, rabbit monoclonal antibodies have a longer affinity maturation time than mice, resulting in higher affinity and specificity. Rabbit monoclonal antibodies also recognize minor epitope variants and cleaved target proteins more effectively.
The rabbit monoclonal antibody has a higher affinity, compared to the murine monoclonal antibody, it can not only affinity with the antigen in the nanomolar range, it can even reach the picomolar level (KD = 10-12M) with a higher sensitivity .
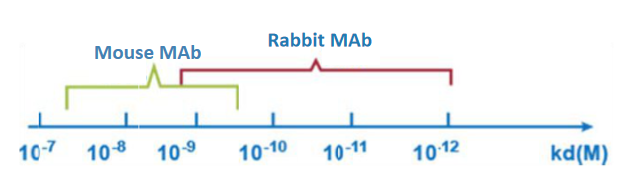
Figure 2. Comparison of affinity of mouse and rabbit antibodies
In terms of detection, since rabbit monoclonal antibodies detect more refined epitopes, there is less chance of cross-reaction with other proteins. At the same time, rabbit monoclonal antibodies bind to target antigens with higher affinity, which means that researchers can perform molecular assays with smaller amounts of antibody and therefore have a higher signal-to-noise ratio compared to mouse monoclonal antibodies.
Immunoglobulin Structure
Rabbits have more IgG as isotype control, while mice have a higher proportion of IgM. Rabbits are more likely to produce specific antibodies due to the better specificity of IgG than IgM. At the same time, the CDR3 loop (third complementary determining region) of the rabbit light chain is significantly longer compared to humans and mice. This property may also contribute to the increased binding capacity of rabbit monoclonal antibodies. In addition, rabbit IgG generally has fewer amino acid residues at the N-terminal and D-E loops. These biomolecules also have unusual structural inter-domain disulfide bonds that may play a critical role in increasing the stability of these molecules and in extending shelf life.
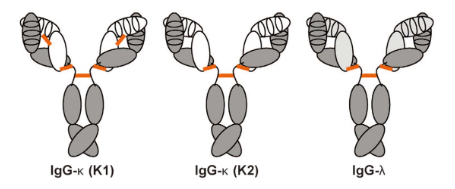
Figure 3. Diagram of natural rabbit IgG
Conclusion
Monoclonal antibodies have traditionally been generated in rodents (e.g., mice) because it is cost effective and easier to administer. However, the immune system of rabbits is capable of producing a broader range of high-affinity antibodies compared to mice, making rabbits an increasingly preferred host species for monoclonal antibodies in a wide range of research, diagnostic and clinical applications. Rabbit monoclonal antibodies can better recognize subtle differences (e.g., epitope variants such as modifications, mutations, conformational changes), detect a broader range of antigens such as small molecules and peptides, and recognize multiple epitopes for each target antigen. Most importantly, rabbit monoclonal antibodies respond better to immunogens and have a higher affinity and specificity for epitopes. Moreover, the experimental results of murine monoclonal antibodies may be confounded by the HAMA effect, which is not the case for rabbit monoclonal antibodies. In the direction of detection, rabbit monoclonal antibody is more sensitive, has lower limit of detection and requires lower sample concentration compared to murine monoclonal antibody.
Reference
1.Feng L, Wang X, Jin H. Rabbit monoclonal antibody: potential application in cancer therapy. American Journal of Translational Research. 2011;3(3):269 .
2.Justus Weber, Haiyong Peng, Christoph Rader. From rabbit antibody repertoires to rabbit monoclonal antibodies. Experimental & Molecular Medicine 2017 (49)
3.Rossi, S. et al. Rabbit monoclonal antibodies: a comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal Am J Clin Pathol. 2005 Aug;124(2):295-302. doi: 10.1309/NR8HN08GDPVEMU08
4.Vilches-Moure, J.G. and Ramos-Vara, J.A., 2005. Comparison of rabbit monoclonal and mouse monoclonal antibodies in immunohistochemistry in canine Journal of veterinary diagnostic investigation, 17(4), pp.346-350.
5.Weber, J. et al. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp Mol Med. 2017; 49(3): e305. doi: 10.1038/emm.2017.23
Join Festival of Biologics USA 2023 with DetaiBio!
The Festival of Biologics brings together pharma & biotech, academics and research institutes, together with their partners across the value chain. Across antibodies, immunotherapy and biosimilars our participants share research, create new partnerships, and tackle the clinical trials, manufacturing and commercial challenges involved in bringing new therapies to market.

Conference Informantionn
Conference Name: Festival of Biologics USA 2023
Conference Date: March 20 – 22, 2023
Conference Venue: San Diego Marriot Hotel & Marina, San Diego, CA
DetaiBio Booth: #29
Conference Agenda

Speakers (partial list)
Whole List: https://www.terrapinn.com/conference/festival-of-biologics-usa/speakers.stm
About Us
Detai Bioengineering Co., Ltd. (“DetaiBio”), established in 2013, is a CRO vendor focusing on antibody discovery and functional protein research file. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
Applications of Nanobodies
Since their discovery, nanobodies have been widely used in many medical research fields such as biopharmaceutical industry, IVD, oncology and immunology research. Nanobodies not only have a potential role in in vivo imaging, but their development as biologic drugs for the treatment of lung diseases, systemic lupus erythematosus, etc. has also seen great development.
Tumor Diagnosis and Definition of Cancer Biomarkers
Nanobodies can effectively penetrate in tumor tissues and can be used as good tracers to assist in early diagnosis and prevention of cancer by detecting or defining biomarkers. Compared to other diagnostic technologies based on monoclonal antibodies, the high stability of nanobodies under extreme temperature, pH or ionic strength ensures their application under harsh conditions. In addition, nanobodies offer higher detection sensitivity and more accurate results.
An ingenious and rapid strategy for generating nanobodies against unknown cancer biomarkers is through immunization of patient samples, by which researchers identified a new breast cancer-specific biomarker, cytokeratin 19 fragment (CYFRA21-1). Similarly, nanobody-based reverse proteomics technology was used for the diagnosis and treatment of glioblastoma multiforme (GBM). Mass spectrometry analysis of nanobody-antigen binding pairs allowed the identification of novel GBM biomarkers TRIM28 and β-actin.
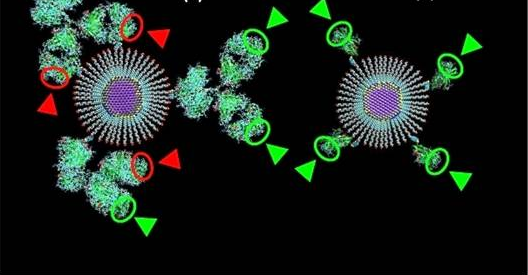
Figure 1. Highly sensitive quantum dot and nanobody coupling product detects HER2, a tumor biomarker for lung and breast cancer
Mediated Drug Delivery and Tumor Targeting Therapy
Nanobodies have a small molecular weight and are able to cross the human blood-brain barrier to the brain, where they can mediate the delivery of drugs, peptides, and other large molecules for therapeutic purposes.
Nanobodies are not affected by the reducing cytoplasmic environment and can be used as in vivo antibodies. On the one hand, some types of nanobodies help to achieve high-resolution protein analysis and better understanding of the relationship between protein structure and function. For example, a nanobody against the SH3 structural domain of the actin branching regulator dermatoglycan suggests that the SH3 structural domain function of dermatoglycan underlies endogenous phase formation and activity. It shows that nanobodies can aid in the discovery or rational design of new therapeutic agents through medicinal chemistry by enabling high-resolution protein analysis. In addition, displacing an overexpressed protein from a cell in molecular studies is relatively easy to achieve, but less so for resident endogenous proteins. However, nanobody has been labeled with a targeting sequence that allows it and the endogenous antigen to be transferred to any compartment or region within the cell, and even if the nanobody fails to bind specifically to the antigen, researchers are able to study the correlation between protein location and function and follow it in real time.
On the other hand, from a therapeutic perspective, one nanobody against CapG, a relatively unknown cytoskeletal protein, substantially curbed breast cancer metastasis in an orthotopic in vivo xenograft model. It suggests that there are many drug targets other than GPCRs or kinases/phosphatases, and by inhibiting these targets the metastatic effects of cancer cells can be diminished, and the future of nanobody applications is bright.
Nanobodies with small molecular weights that target tumor-specific receptors can be used as carriers to deliver toxins or drugs to tumors, thereby reducing non-specific toxicity to normal cells and decreasing side effects. Nanobodies lacking Fc tails also do not trigger Fc-related immunogenic responses when mediating drug transport to tumor cell receptors. Carriers generally used for nanoparticle drugs include liposomes, micelles, albumin-based nucleic acid nanoparticles (NANAPs) and polymer-based aggregates or polymers, which are widely used and effective. Due to the abundance of protein in serum, NANAPs are more biocompatible and safe. Encapsulation of multi-kinase inhibitor 17,864 in EGa1-coated NANAPs allows the lysosomes to digest the NANAPs particles through cytocytosis, allowing the inhibitor to be released in cytoplasmic solution and inhibit the proliferation of cancer cells. In addition, nanobody-mediated artificial aggregates and polymorphs composed of PEGylated PEI conjugates modified with anti-MUC1 nanobodies have also received considerable attention in recent years for targeted therapies.
Nanobody-targeted radionuclide therapy is one of the most widely used modalities to treat cancer. VHH with radiolabel identifies and binds the primary tumor site to the diseased tissue, directly increasing the radiation dose of radionuclide targeted to the tumor, which can significantly reduce the impact and damage to the surrounding normal tissues. Another way to treat cancer is photodynamic therapy, which produces fewer side effects. It is a precise and effective treatment by combining photosensitizers with nano-antibodies to target tumor tissues, and by activating the photosensitizers to trigger a photochemical reaction to produce a cytotoxic effect that kills tumor cells.
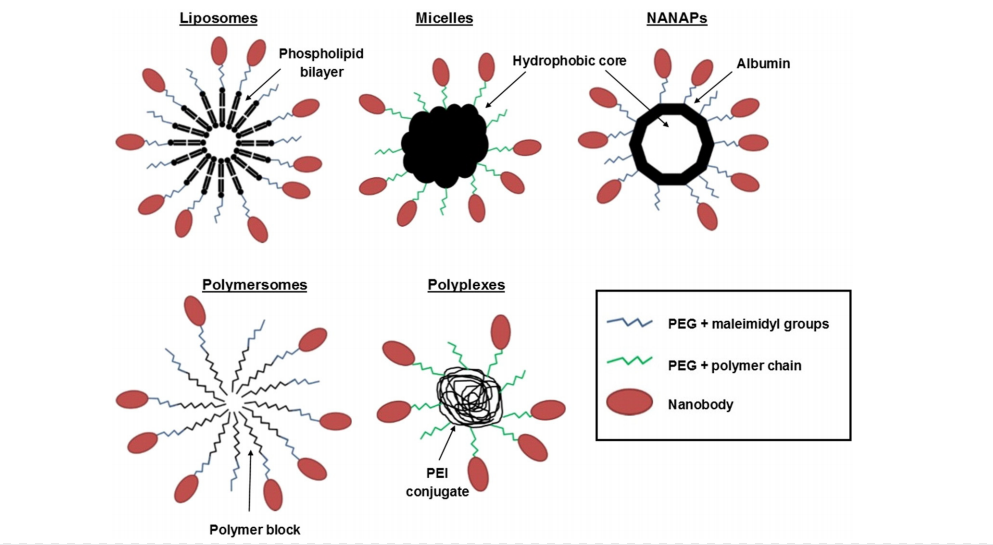
Figure2. Carriers generally used for nanoparticle drugs
Molecular Imaging
In the field of molecular imaging, small molecular weight nanobodies are very advantageous. They enable rapid aggregation and uniform distribution of tumors and are rapidly cleared in the blood, helping to improve the tumor-to-background ratio. In addition, nanobodies can easily bind to several imaging agents and possess relatively safe and high specificity.
Nanobodies are mainly used in molecular imaging for tumor-specific visualization, detection of atherosclerotic plaques, efficacy assessment and individualized therapy. The main modalities for visualization of cancer include single photon emission computed tomography (SPECT), positron emission tomography (PET), ultrasound imaging and optical tumor imaging.
In studying the correlation between labeled nanobodies and the load during tumor treatment, molecular imaging can visualize changes at the molecular level in disease treatment, which facilitates the assessment of tumor efficacy in favor of subsequent treatment.
Research and Treatment of Infectious Diseases
Nanobodies are not only suitable for the diagnosis and treatment of oncological diseases, but also have considerable potential advantages for some infection such as viruses, bacteria, parasites and toxins. Unlike conventional antibodies that cannot protect against viruses that have already invaded host cells, nanobodies not only antagonize viruses at an early stage, but can also be designed as intracellular antibodies to inhibit the replication, assembly and release of viruses. Nanobodies can also be developed for bacterial infections that are primarily treated with antibiotics, avoiding to some extent the problems associated with antibiotic resistance.
For parasites and fungi, nanobodies are currently focused on the treatment of African trypanosomiasis, or sleeping sickness. Thanks to their low molecular weight and high affinity, nanobodies are able to bind to conserved antigenic epitopes hidden by variant glycoproteins on the trypanosome surface membrane, thus interfering with trypanosome endocytosis and exhibiting direct trypanosome lysis.
Due to their antifungal effect, nanobodies are also quite widely used in anti-dandruff shampoos. In addition to this, the use of antibody drugs for antagonizing animal toxins such as snake venom toxins and bacterial toxins has been used and is expanding.
Relative Services
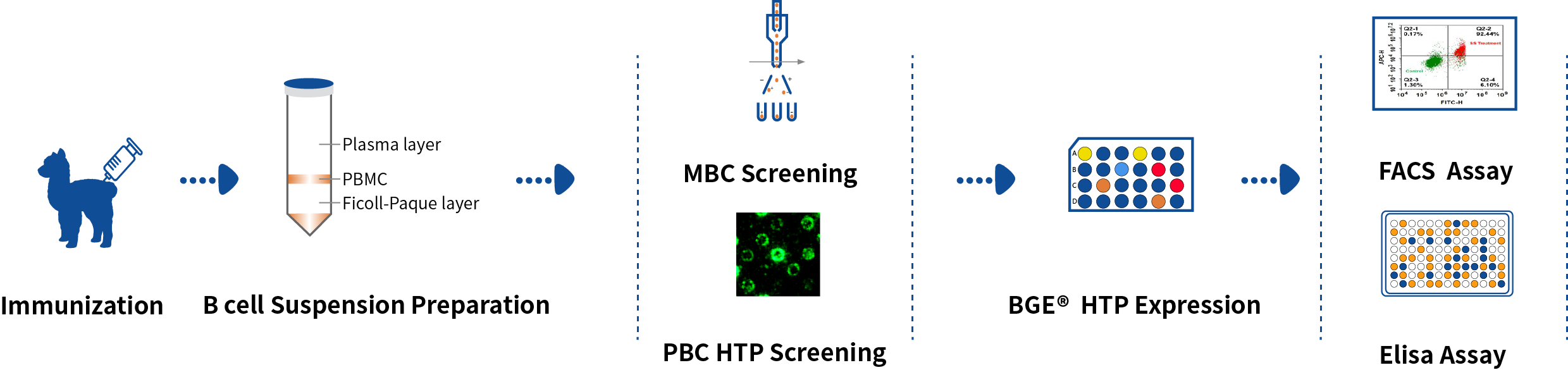
DetaiBio offers SingleB® Alpaca VHH/Nanobody Discovery Service, directly screening B cells. Dual pathway sorting of MBC and PBC to accelerate your nano-antibody discovery, guarneteeing nanobody diversity and affinity, with one-stop service from antigen preparation to antibody expression.
Reference
[1]Isabel Van Audenhove, Jan Gettemans. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer[J]. EBioMedicine,2016,8(C).
[2]D'Huyvetter Matthias, Xavier Catarina,Caveliers Vicky, Lahoutte Tony,Muyldermans Serge, Devoogdt Nick. Radiolabeled nanobodies as theragnostic tools in targeted radionuclide therapy of cancer.[J]. Expert opinion on drug delivery,2014,11(12).
Antibody Humanization
Antibody humanization is an important part of research in the production and preparation of recombinant antibodies (monoclonal antibodies). Antibody humanization is the process of development from murine antibodies to human antibodies. More than a hundred years ago, the revelation of the principles of specific binding of antibodies to antigens and passive immunological properties of antibodies opened up new ways of disease diagnosis. The introduction of monoclonal antibody technology in 1975 accelerated the widespread use of this method. Initially, most of the monoclonal antibodies used in clinical practice were mouse-derived monoclonal antibodies, and there were various limitations in the use of mouse-derived antibodies due to the specificity of human and mouse species. Although murine antibodies are specific to the target antigen and can bind specifically to the target antigen, they cannot activate the corresponding human effector systems, such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), so that antigen-antibody reactions cannot occur normally. On the other hand, murine antibodies which enter the human body, may be seen as exogenous proteins, which can cause the human immune system to respond and produce specific antibodies using mouse antibodies as antigens, i.e. human anti-mouse antibody (HAMA), which is usually cleared quickly in the human body with a short half-life. Because of the limitations of mouse-derived antibodies in clinical applications, humanization of mouse-derived antibodies has been carried out using recombinant DNA technology to humanize the antibodies.
Principle of Antibody Humanization
Humanization of mouse-derived antibodies is to genetically modify them to have an extremely similar profile to human antibody, thus evading recognition by the human immune system and avoiding the induction of HAMA. Two basic principles should be followed when performing antibody humanization: 1) to maintain or improve the affinity and specificity of the antibody. 2) to greatly reduce or largely eliminate the immunogenicity of the antibody. The antibody humanization process goes through three stages: chimeric antibody, humanized antibody and human antibody. Depending on the principle of action, humanized antibodies can be divided into four categories: chimeric antibody, reshaped antibody, resurfacing antibody and fully humanized antibody.
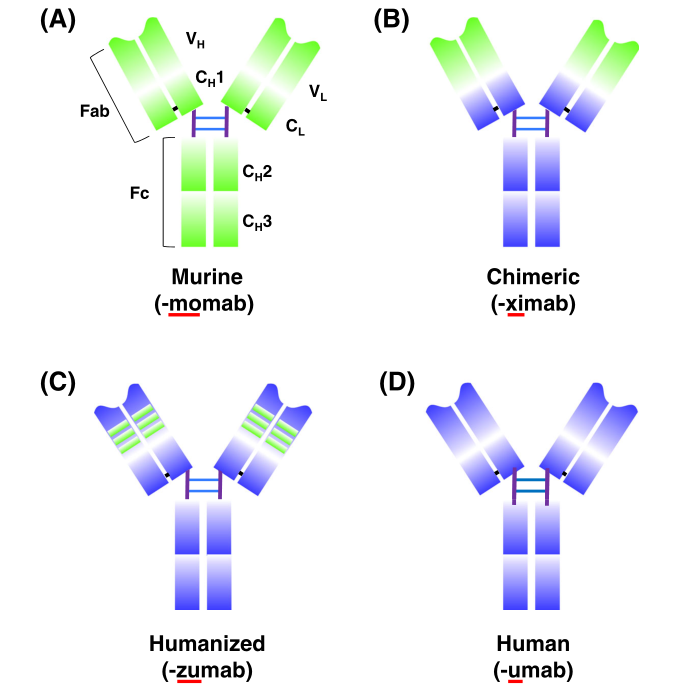
Figure 1. Schematic overview of mouse antibodies (green structural domain) to fully human antibodies (orange structural domain) and related antibody humanization. a Mouse-derived monoclonal antibodies. b Chimeric monoclonal antibodies: variable region is mouse-derived, remaining chains are human-derived. CH: heavy chain constant region; CL: light chain constant region; Fab and Fc: fragments resulting from proteolysis; VH: heavy variable region; VL: light chain variable region.【1】
Process of Human Antibody Production
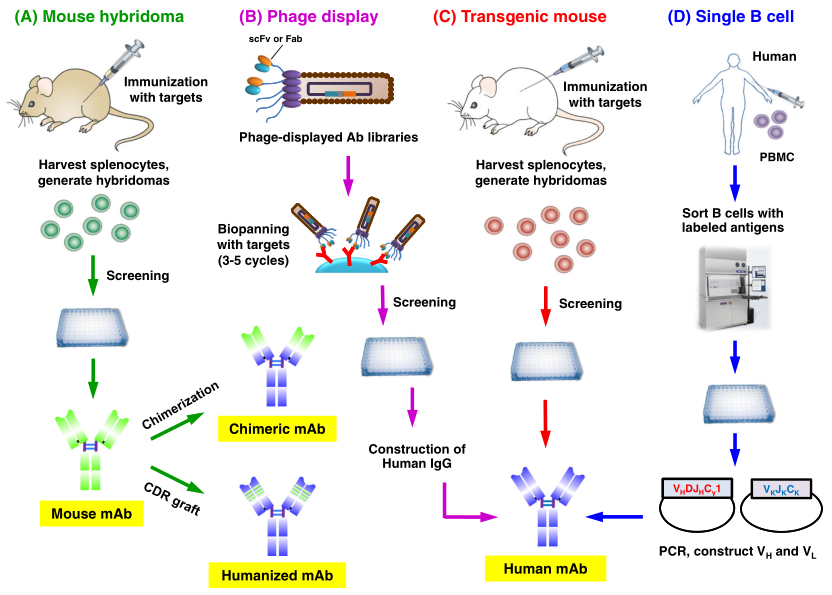
Figure 2. Humanized Antibody Discovery Methods
Clinical Applications of Humanized Antibody
In recent years, the emergence of humanized antibodies and fully human antibodies has brought new hope for clinical applications in the treatment of oncology, autoimmune diseases and cardiovascular diseases, as well as anti-transplant rejection and antiviral infections. These include reducing the adverse effects of traditional oncology drug therapy, acting as a tumor-specific marker, minimizing and avoiding rejection that occurs after transplantation, protecting the function of transplanted organs, and acting as an induction for solid organ transplantation therapy. The first chimeric Fab antibody-Abciximab (ReoPro) was approved by the US FDA in 1994 and has been widely used in the treatment of various cardiovascular diseases, mainly for the prevention of restenosis after coronary artery formation surgery. Recent studies have shown that humanized antibodies have promising applications in antiviral infections (e.g. HBV, HCV, HIV and other viral infectious diseases).
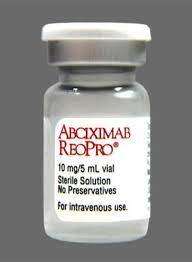
Figure 3. Abciximab (ReoPro)
Relative Services
DetaiBio offers One-stop service from antibody discovery to antibody humanization. By self-developed SingleB® mAb discovery platformand BGE® antibody HTP expression platform, the timeline of antibody discovery and expression can be reduced to 49 days and 14 days, respectively. DetaiBio also offers antibody humanization service to ensure that the delivered antibodies have sufficient drug-forming capacity.
Reference
1.Rossotti MA, Bélanger K, Henry KA, Tanha J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022 Jul;289(14):4304-4327. doi: 10.1111/febs.15809. Epub 2021 Mar 25. PMID: 33751827.
2.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020 Jan 2;27(1):1. doi: 10.1186/s12929-019-0592-z. PMID: 31894001; PMCID: PMC6939334.
Join Discovery On Target 2022 with DetaiBio!
Discovery on Target (DOT) highlights advances in current and emerging “hot” targets and technologies, as well as target validation strategies for the discovery and development of novel therapeutic agents ranging from biologics to small molecules.
The 2022 Annual event brings back popular topics like PROTACs and RNA plus new programming on immunomodulation, antivirals, neurodegeneration targets, KRAS, and AI.

Conference Informantion
Conference Name: Discovery On Target 2022
Conference Date: October 17-20, 2022
Conference Venue: Sheraton Boston - Plaza Level Grand Ballroom, Boston, MA
DetaiBio Booth: #611
Conference Agenda

Speakers (partial list)

Whole List: https://www.discoveryontarget.com/speaker-biographies
About Us
Detai Bioengineering Co., Ltd. (“DetaiBio”), established in 2013, is a CRO vendor focusing on antibody discovery and functional protein research file. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us
49 Days Monkeypox Virus Antibody Discovery with DetaiBio SingleB® Platform
Since May 2022, monkeypox outbreaks have occurred in several countries around the world. According to the CDC, as of August 26th 2022, there have been more than 47,000 cases of monkeypox worldwide, involving 99 countries, including the United States, the United Kingdom, and others (Figure 1).
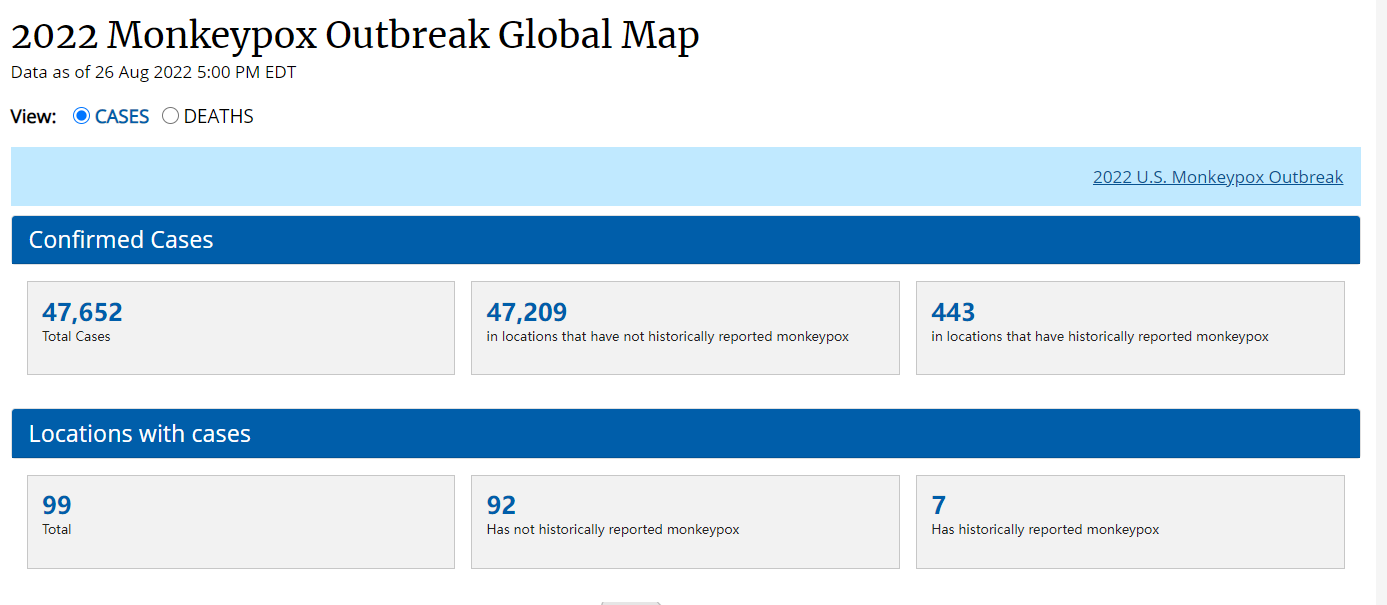
Figure 1: CDC Data of Monkeypox Outbreak Globally
About Monkeypox
Monkeypox, also known as monkey smallpox, has been designated as a zoonotic disease that originated in the tropical rainforest region of West and Central Africa. The pathogen of monkeypox is monkeypox virus (MPXV), which was first identified in 1958 in experimental crab-eating monkeys (Figure 2).
Monkeypox virus is transmitted primarily through animals, with people becoming infected after being bitten or in close contact with an animal carrying the virus, and through blood, body fluids and contaminants. Patients with monkeypox are contagious at the onset of symptoms.
The most important symptom of monkeypox virus infection is the skin rash. The rash can vary in number from a few to several thousand and tends to be concentrated on the face, palms and soles of the feet, and can also be found in the mouth and eyes.
Figure 2: Monkeypox Observed under the Microscope
DetaiBio Facilitates Fast Discovery of MPXV Antibodies by SingleB® Platform
Through the self-built SingleB® Fast mAb discovery platform, DetaiBio can obtain nearly 100 MPXV antibodies in 49 days, involving 4 key epitopes, A35R, B6R, A29L and M1R, which significantly improves the efficiency of monoclonal antibody discovery, saving time for antibody development. Detaibio preferentially selected eukaryotic expression of monkeypox antigens to immunize mice and directly screened monkeypox antigen-specific memory B cells and plasma cells from peripheral blood, obtaining antibodies with natural maturation and rich sequence diversity in vivo.

Based on the customer's requirements, DetaiBio can develop a sandwich antibody pair suitable for their specific application requirements, making it applicable to ELISA kit development, colloidal gold test strip development and other researches IVD field.
B Cell Double Screening
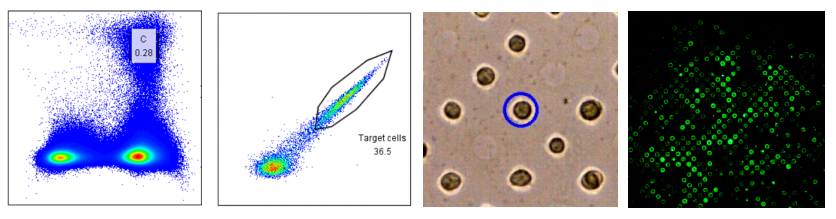
Results of FACS screening of MBC and DeepLight® screening of PBC
ELISA Assay of Developed MPXV Antibodies
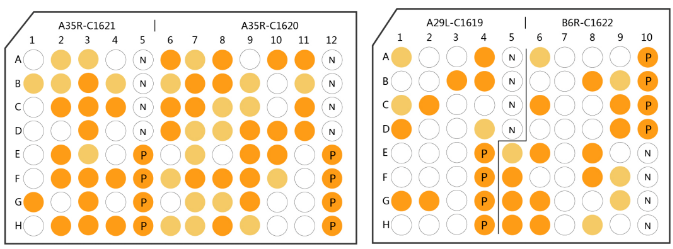
A35R-C1620 Antibodies Diversity

About Us
Detai Bioengineering Co., Ltd. (“DetaiBio”), established in 2013, is a CRO vendor focusing on antibody discovery and functional protein research file. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
DetaiBio Sincerely Welcome You to Join ADC San Diego 2022
13th World Antibody-Drug Conjugate Conference (ADC) will be held in San Diego, USA from September 6-9, 2022. The conference fouces on ADCs in combination, the next generation of payloads, the use of biomarkers in clinical development, as well as how to evolve manufacturing processes to mitigate supply chain challenges. With many professionals gathered in San Diego, we would like to invite you to attend this conference and to communicate with us at the venue.

Conference Informantion
Conference Name: Antibody-Drug Conjugate Conference
Conference Date: September 6-9, 2022
Conference Venue: Sheraton San Diego Hotel and Marina, 1380 Harbor Island Dr, San Diego, CA 92101, United States
DetaiBio Booth: #21
Conference Agenda
Speakers (partial list)


Whloe List: https://worldadc-usa.com/scientific-program/speakers/
About Us
Detai Bioengineering Co., Ltd. (“DetaiBio”), established in 2013, is a CRO vendor focusing on antibody discovery and functional protein research file. DetaiBio is aiming to provide high quality and economic offer service to speed up life science for our client in different fields, such as antibody drug discovery, in-vitro diagnosis and academic research.
The main services offer by DetaiBio:
——SingleB® for antibody/VHH discovery service
——High-throughput recombinant antibody expression service (2 week delivery)
——Hybridoma sequencing service
——Customized protein expression service ( E.coli, Mammalian)
Follow Us

Facebook

Twitter
Customer Published Literature
| Sun JH, Huang M, Fang Z, et al. Nerve bundle formation during the promotion of peripheral nerve regeneration: collagen VI-neural cell adhesion molecule 1 interaction[J]. Neural Regeneration Research. 2022, 17(5):1023-1033. |
| Journal | Neural Regeneration Research | IF(2021) | 5.135 |
| doi | 10.4103/1673-5374.324861. | Product | NCAM120 |
| Services | Recombinant Protein Production | System | Mammalian Expression |
| The DNA segment encoding the extracellular region of Ig-like domains (Leu20 to Val495) and that encoding the extracellular region of FnIII domains (Gln496 to Gly740) of human NCAM120 were inserted between the BglII and XhoI sites of the mammalian expression system vector proEM™ (DetaiBio, Nanjing, China) |
| Luo ZH, Liao T, Zhang YN, et al. Ex vivo anchored PD‐L1 functionally prevent in vivo renal allograft rejection[J]. Bioengineering & Translational Medicine. 2022 |
| Journal | Bioengineering & Translational Medicine | IF(2021) | 10.711 |
| doi | 10.1002/btm2.10316 | Product | PD-L1 + APT542 |
| Services | Recombinant Protein Production | System | Mammalian Expression |
| The protein amino acid sequence of rat-derived PD-LI and anchoring structure APT542 were codon optimized using MaxCodonTM Optimization Program (V13) (DetaiBio). The PD-L1 + APT542 gene was inserted into the expression vector pcDNA3.1(-) by wholegenome synthesis and double enzyme digestion. Accuracy of the final expression vector was confirmed by restriction enzyme digestion and sequencing. Finally, the vector was transformed into DH5a cells, and the plasmid was extracted using a plasmid extraction kit. The plasmid was then transfected into mammalian HEK293 cells for transient expression, and the fusion protein map-PD-L1 was purified using affinity chromatography. |
| Xu WF, Che Y, Zhang Q, et al. Apaf-1 Pyroptosome Senses Mitochondrial Permeability Transition[J]. Cell metabolism. 2021, 33(2):424-436. |
| Journal | Cell metabolism | IF(2021) | 27.287 |
| doi | 10.1016/j.cmet.2020.11.018 | Product | CASPASE-4 |
| Services | Recombinant Protein Production | System | Mammalian Expression |
Human CASPASE-4-pcDNA3.1(-)-AMP-His(Detai Bio)
Human CASPASE-4-pcDNA3.1(-)-AMP-HA(Detai Bio)
Human CASPASE-4(1-90AA)-pcDNA3.1(-)-AMP-His(Detai Bio)
Recombinant pro-caspase-4-eGFP-His(Detai Bio)
Recombinant pro-caspase-11-eGFP-His(Detai Bio)
Recombinant caspase-4 (CARD)-Fc-HA(Detai Bio) |
| Sun BQ, Xue YF., Du XL, et al. Identification of genetic determinants of hemolytic activity of Riemerella anatipestifer using random transposon mutagenesis[J]. Veterinary research, 2021(52), 19. |
| Journal | Veterinary research | IF(2021) | 3.699 |
| doi | 10.1186/s13567-021-00900-6 |
| Services | Protein purification |
| The soluble recombinant proteins rRiean_0317, rRiean_0653, and rRiean_1143 were purified by Ni-iminodiacetic acid affinity chromatography (Detai Bio-Tech (Nanjing) Co., Ltd., Nanjing, China) |